0121
REnal Flow and Microstructure AnisotroPy (REFMAP) MRI in Normal and Peritumoral Renal Tissue1NYU School of Medicine, NYU Langone Medical Center, New York, NY, United States, 2Radiology, NYU Langone Medical Center, New York, NY, United States, 3Center for Advanced Imaging and Innovation (CAIIR), New York, NY, United States, 4Urology, NYU Langone Medical Center, New York, NY, United States
Synopsis
Using a recently developed joint intravoxel incoherent motion (IVIM)- diffusion tensor imaging (DTI) protocol for kidney evaluation, we present reproducibility analysis of its metrics in normal volunteers, as well as pilot assessments in several patients with renal masses prior to surgery. Reproducibility analysis indicates a subset of robust parameters, including structural and microcirculation markers in both cortex and medulla, for clinical application. Preliminary results in renal mass patients suggest multifactorial differences from controls, supporting the need for advanced diffusion characterization in assessing renal functional reserve.
Purpose
Renal dysfunction occurring following a renal insult, such as surgical resection, is a complex interplay of microstructural and microvascular phenomena. Currently, the standard assessment of renal function is estimated glomerular filtration rate (eGFR), which is calculated from serum creatinine levels. eGFR is an indirect global measure of kidney function and cannot assess lateral response (operated vs. contralateral kidney), differentiate between pathophysiologies, or predict recovery. Diffusion-weighted imaging (DWI) techniques have attempted to tease apart complexities of renal tissue1. Intravoxel incoherent motion (IVIM)2, which distinguishes tubular/vascular flow from microstructure, is sensitive to renal allograft rejection3,4 and vascularity / cellularity of renal masses5-8. Diffusion tensor imaging (DTI)9 measures water diffusion anisotropy, which is decreased in diabetic nephropathy10, allograft dysfunction11,12, ischemia-reperfusion damage13 and chronic parenchymal disease14. We have recently demonstrated a joint IVIM-DTI approach which allows assessment of the directional anisotropy of both the fast pseudodiffusion (vascular / tubular flow) and the slow tissue diffusion (microstructure) components15, a topic which has gained recent attention for renal imaging16-18. We present reproducibility of joint IVIM-DTI in normal volunteers and pilot data in renal mass patients.Methods
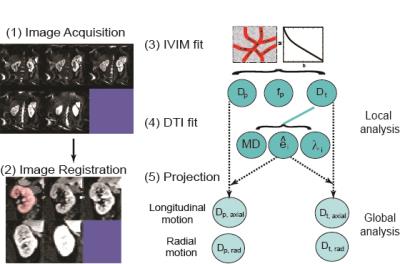
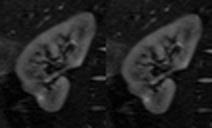
10 volunteers and 3 patients were examined using a wide-bore 3T-scanner (VERIO, Siemens Healthcare, Erlangen, Germany). Volunteers were examined twice in a time interval of 39.4 +/- 37.1 days. Figure 1 shows the workflow for this study. IVIM-DTI was acquired during free-breathing using a prototype twice-refocused spin echo Echo-Planar-Imaging (EPI) sequence with reversed slice gradient polarity fat suppression19: 2 averages; b-values 0, 10, 30, 50, 80, 120, 200, 400, 600, 800 s/mm2; 20 diffusion directions; slice thickness=6 mm; voxel 2.2×2.2×6 mm3, acquisition time 15 minutes. Retrospective 2D affine registration of EPI images was performed separately for the right and left kidney with inhouse software (FireVoxel) (Figure 2)20, with rejection of manually inspected images with extreme signal loss. IVIM-DTI was analyzed with custom code (IgorPro, Wavemetrics, Inc., Portland, OR, USA). A median (nonlinear edge preserving) filter of 3×3 voxels was applied. A cylindrical two-compartment description quantified the global anisotropies of the diffusion and perfusion components15. A tensor was fitted to the structural diffusivities (Dt), and a scalar perfusion fraction was constrained as the average of all directional values. Directional pseudodiffusivities (Dp) were determined. Dt and Dp from all cortex or medullary voxels and directions were ‘projected’ along the structural primary tensor axis (21,22). Global Dt and Dp anisotropies were estimated assuming cylindrical symmetry (λ2=λ3). The parameter set is termed Renal Flow and Microstructure AnisotroPy (REFMAP) MRI. Averages over regions of interest segmenting cortex and medulla tissue drawn on central slices using b0 and FA maps were computed. Reproducibility of all REFMAP-MRI metrics was assessed with intra-class correlation coefficient (ICC) and intra-subject coefficient of variation (CV) values. REFMAP-MRI metrics in renal mass patients were separately computed in tumor-bearing (ipsilateral) and contralateral kidneys and compared with eGFR.Results
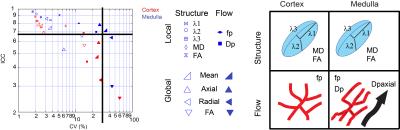
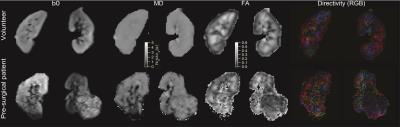
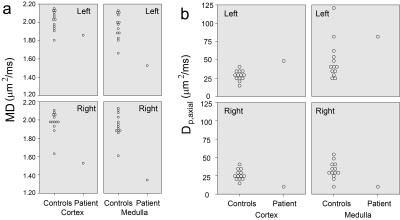
Figure 2 shows an animation of a set of single kidney slices without and with registration, showing improved organ alignment prior to IVIM-DTI processing. Figure 3 shows a cross-plot of ICC and CV values for REFMAP-MRI parameters in volunteers, stratified by local / global analysis, flow / structure compartment, and cortex / medulla. Structural parameters are more reproducible than flow parameters, local more than global, and medullary more than cortical. Figure 4 shows REFMAP-MRI maps in a pre-surgical renal mass patient with normal eGFR but with proteinuria due to diagnosis of minimal change renal disease along with analogous maps from a healthy volunteer. REFMAP-MRI comparisons shown in Figure 5 highlight the differences between values in this patient and the normal group results; structural diffusivities are uniformly lower in both ipsilateral (left) and contralateral (right) kidney for this patient. Conversely, the global Dpaxial value is higher ipsilaterally and lower contralaterally than controls.Discussion
The ICC/CV analysis identifies a subset of reproducible parameters (Figure 3) (ICC >0.7 and CV < 30%) for further use, which includes the full structural tensor and average perfusion fraction in cortex and medulla, as well as medullary average and global axial pseudodiffusion Dp. This robust parameter set can be carried forward to clinical applications. The patient results shown in Figure 4 reflect complex pathophysiology. The reduced MD values may reflect a global degradation of tissue integrity or fibrotic deposition. The mixed changes in global axial pseudodiffusion Dp may reflect tubular flow alterations as the renal tissue responds to the mass with compensatory redistribution of function. This complex response exemplifies the need for the multi-level view provided by REFMAP-MRI as opposed to global eGFR only, and portends applicability in predicting renal reserve against surgical shock.Acknowledgements
We acknowledge Thorsten Feiweier (Siemens Healthcare) for providing support of our use of the prototype diffusion-weighted imaging sequence employed in this study.References
1. Sigmund EE, Vivier PH, Sui D, Lamparello NA, Tantillo K, Mikheev A, Rusinek H, Babb JS, Storey P, Lee VS, Chandarana H. Intravoxel Incoherent Motion and Diffusion-Tensor Imaging in Renal Tissue under Hydration and Furosemide Flow Challenges. Radiology 2012;263(3):758-769.
2. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161(2):401-407.
3. Eisenberger U, Thoeny HC, Binser T, Gugger M, Frey FJ, Boesch C, Vermathen P. Evaluation of renal allograft function early after transplantation with diffusion-weighted MR imaging. Eur Radiol 2010;20(6):1374-1383.
4. Blondin D, Lanzman RS, Klasen J, Scherer A, Miese F, Kropil P, Wittsack HJ. Diffusion-attenuated MRI signal of renal allografts: comparison of two different statistical models. AJR Am J Roentgenol 2011;196(6):W701-705.
5. Chandarana H, Lee VS, Hecht E, Taouli B, Sigmund EE. Comparison of biexponential and monoexponential model of diffusion weighted imaging in evaluation of renal lesions: preliminary experience. Invest Radiol 2011;46(5):285-291.
6. Rheinheimer S, Stieltjes B, Schneider F, Simon D, Pahernik S, Kauczor HU, Hallscheidt P. Investigation of renal lesions by diffusion-weighted magnetic resonance imaging applying intravoxel incoherent motion-derived parameters--initial experience. Eur J Radiol 2012;81(3):e310-316.
7. Chandarana H, Kang SK, Wong S, Rusinek H, Zhang JL, Arizono S, Huang WC, Melamed J, Babb JS, Suan EF, Lee VS, Sigmund EE. Diffusion-Weighted Intravoxel Incoherent Motion Imaging of Renal Tumors With Histopathologic Correlation. Invest Radiol 2012.
8. Gaing B, Sigmund EE, Huang WC, Babb JS, Parikh NS, Stoffel D, Chandarana H. Subtype Differentiation of Renal Tumors Using Voxel-Based Histogram Analysis of Intravoxel Incoherent Motion Parameters. Invest Radiol 2014.
9. Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. Nmr in Biomedicine 1995;8(7-8):333-344.
10. Lu L, Sedor JR, Gulani V, Schelling JR, O'Brien A, Flask CA, Macrae Dell K. Use of diffusion tensor MRI to identify early changes in diabetic nephropathy. Am J Nephrol 2011;34(5):476-482.
11. Hueper K, Gutberlet M, Rodt T, Gwinner W, Lehner F, Wacker F, Galanski M, Hartung D. Diffusion tensor imaging and tractography for assessment of renal allograft dysfunction-initial results. Eur Radiol 2011;21(11):2427-2433.
12. Lanzman RS, Ljimani A, Pentang G, Zgoura P, Zenginli H, Kropil P, Heusch P, Schek J, Miese FR, Blondin D, Antoch G, Wittsack HJ. Kidney transplant: functional assessment with diffusion-tensor MR imaging at 3T. Radiology 2013;266(1):218-225.
13. Cheung JS, Fan SJ, Chow AM, Zhang J, Man K, Wu EX. Diffusion tensor imaging of renal ischemia reperfusion injury in an experimental model. Nmr in Biomedicine 2010;23(5):496-502.
14. Gaudiano C, Clementi V, Busato F, Corcioni B, Orrei MG, Ferramosca E, Fabbri E, Berardi P, Santoro A, Golfieri R. Diffusion tensor imaging and tractography of the kidneys: assessment of chronic parenchymal diseases. Eur Radiol 2013;23(6):1678-1685.
15. Notohamiprodjo M, Chandarana H, Mikheev A, Rusinek H, Grinstead J, Feiweier T, Raya JG, Lee VS, Sigmund EE. Combined intravoxel incoherent motion and diffusion tensor imaging of renal diffusion and flow anisotropy. Magn Reson Med 2015;73(4):1526-1532.
16. Hilbert F, Bock M, Neubauer H, Veldhoen S, Wech T, Bley TA, Kostler H. An intravoxel oriented flow model for diffusion-weighted imaging of the kidney. NMR Biomed 2016;29(10):1403-1413.
17. van Baalen S, Leemans A, Dik P, Lilien MR, Ten Haken B, Froeling M. Intravoxel incoherent motion modeling in the kidneys: Comparison of mono-, bi-, and triexponential fit. J Magn Reson Imaging 2016.
18. Ye Q, Chen Z, Zhao Y, Zhang Z, Miao H, Xiao Q, Wang M, Li J. Initial experience of generalized intravoxel incoherent motion imaging and diffusion tensor imaging (GIVIM-DTI) in healthy subjects. J Magn Reson Imaging 2016;44(3):732-738.
19. Deshpande VS, Wisner DJ, Grinstead JW, Feiweier T, Joe BN, Laub GA. Technical advances for breast diffusion MR imaging on wide-bore 3T systems. Proceedings ISMRM 2011; Montreal, Canada. p 3076.
20. Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B. Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast-enhanced MRI alone and in combination: preliminary experience. Journal of magnetic resonance imaging : JMRI 2010;31(3):589-600.
21. Jones DK, Basser PJ. "Squashing peanuts and smashing pumpkins": how noise distorts diffusion-weighted MR data. Magn Reson Med 2004;52(5):979-993.
22. Hirsch JG, Schwenk SM, Rossmanith C, Hennerici MG, Gass A. Deviations from the diffusion tensor model as revealed by contour plot visualization using high angular resolution diffusion-weighted imaging (HARDI). Magma 2003;16(2):93-102.
Figures