0119
5D MRI for late enhancement dynamics in lung fibrosis1Department of Radiology, Papworth Hospital NHS Foundation Trust, Cambridge, United Kingdom, 2Department of Diagnostic and Interventional Radiology with Nuclear Medicine, Thoraxklinik at Heidelberg University Hospital, Heidelberg, Germany, 3Institute for Clinical Radiology, Ludwig-Maximilians-University Hospital Munich, Munich, Germany, 4Medical Physics in Radiology, German Cancer Research Center, Heidelberg, Germany, 5Siemens Healthcare, Erlangen, Germany, 6Joint Department of Physics, The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, London, United Kingdom
Synopsis
We analyzed the contrast agent late dynamics in fibrotic lung lesions of different severity, assessed with perfusion MRI, using a 5D reconstruction (respiratory 4D at different time points) of a 3D radial stack-of-stars spoiled gradient echo sequence with golden-angle spacing acquired in free-breathing. Idiopathic pulmonary fibrosis (IPF) shows late peak enhancement regardless of severity level and possibly no wash-out within the scan interval, while
Purpose
To analyze late contrast agent (CA) dynamics in fibrotic lung lesions using a 5D reconstruction of radial gradient echo MRI data acquired in free-breathing.Methods
20 patients (mean[interquartile] age: 71[61-73] years; male:female 13:7) with diagnosis of idiopathic pulmonary fibrosis (IPF, n=12) and non-IPF (idiopathic or connective tissue disease-related nonspecific interstitial pneumonia, n=8) underwent MRI of the lung at 1.5T (Magnetom Aera, Siemens Healthcare, Erlangen). CA was administrated (0.07mmol/kg, 4-5 ml/s injection rate) to assess pulmonary perfusion using a standard first-pass perfusion acquisition. Starting two minutes (121.5±7.2 s) after CA injection, a prototype 3D radial stack-of-stars spoiled gradient echo sequence with golden-angle spacing1,2 was acquired sagittally in free-breathing for 350 s (FOV: 385×385×300 mm, voxel size: 1.5×1.5×5.0 mm, TR/TE: 3.77/1.69 ms, flip angle: 12°, readout BW: 490 Hz/px, partial Fourier: 0.75, 33% slice oversampling, radial views: 2035, reduced slice resolution: 0.6).
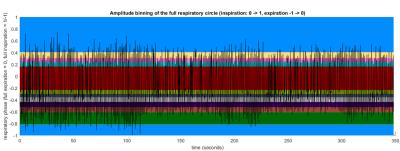
Data was preprocessed in MATLAB 2015a (The MathWorks, Inc., Natick, MA). Respiratory self-gating based on the magnitude signal of the k-space center3 was performed, where the first seven angles were excluded to account for the MRI signal reaching a steady-state. A linear correction based on the exhale peaks of the surrogate signal was applied to account for drifts resulting from CA wash-out. Starting from end-expiration spokes were assigned into 11 overlapping respiratory bins distinguishing between inspiration and expiration (Fig. 1).
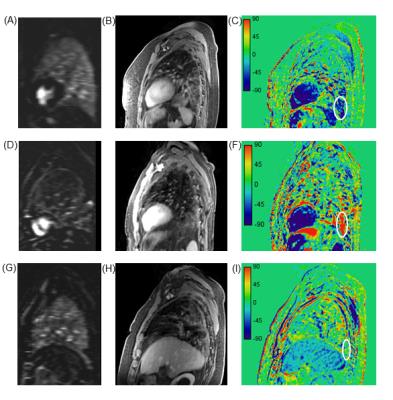
The radial MRI raw data was then further assigned to 5 overlapping time-steps (separated by 58.1s each). After coil sensitivity profiles were jointly estimated for the whole data set4, 5D images (11 respiratory phases, 5 time steps) were reconstructed separately for each time-step using the joint 4D MoCo-HDTV algorithm5. For each patient the time-course of the signal in fibrotic lesions was evaluated in 10 adjacent slices on the reconstructed end-expiratory phase. Time of peak intensity, wash-in and wash-out rates were obtained by a linear least squares fit. The severity of perfusion defect was evaluated by a radiologist on subtraction perfusion maps (FIG. 2 A,D,G). Exemplary images at the first time point (FIG. 2 B,E,H) and signal change maps (FIG 2 C,F,I) are shown for 3 patients exhibiting wash-out, wash-in and plateau behavior. Statistical analysis was performed using R statistical software version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
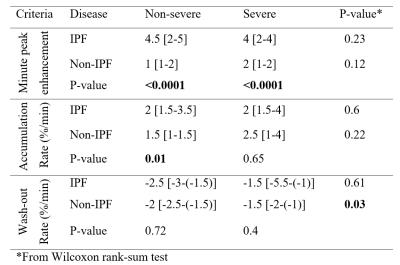
IPF displays later peak enhancement at both severity levels, p<0.0001, and higher accumulation rate in non-severe disease, p<0.01, as compared to non-IPF. In non-IPF, fibrotic lesions have fast time to peak and slow accumulation rate; however, the lesions exhibiting severe perfusion defects had slower wash-out rate as compared to non-severe ones, p<0.05. In IPF, fibrotic lesions have late peak enhancement and slow accumulation rate, with no significant difference between severity levels. Continuous CA accumulation, without wash-out, was found only in patients with IPF. We found CA accumulation rate of 1.5 [0.8-3]%/minute and a wash-out rate of -1.5 [-2.5-(-0.6)]%/minute. 44/200 (22%) lesions, in one IPF and 3 non-IPF cases, demonstrated plateau signal intensity, with a rate of signal intensity change between [-0.6-0.8]%/minute.Discussion
Our results extend the findings of 6 that signal in inflammatory lesions peaks in the initial dynamic phase at 1 minute, while fibrotic lesions have delayed peak enhancement between 3-9 minutes. We had 4 cases with plateau signal: two non-IPF, which showed normal perfusion, where we suspect that the peak enhancement occurred before the start of the stack-of-stars acquisition and these fibrotic lesions had a fast wash-out, similar to that of inflammatory lesions; and two patients, one with IPF and one with non-IPF, with severe perfusion defects, where we assume that only minimal, if at all, CA reached those advanced fibrotic lesions.
The early enhancement pattern of non-severe lesions in non-IPF may be due to an increase of neovascularization, which decreases in advanced lesions7. In IPF, well-capillarized intraluminal lesions are usually not present and severe fibrosis was reported to have lower vascularity in fibroblastic foci8. This might explain our findings of late peak enhancement in IPF and the early one in non-IPF. In pulmonary fibrosis, vascular proliferation in bronchial vessels, leads to increased contribution of systemic blood flow to lung perfusion9. This fact may have contributed to the slow accumulation and wash-out predominant in non-IPF patients.
Conclusions
The timing of CA accumulation is influenced by the type and severity of lung fibrosis. Results obtained with the presented non-invasive, non-irradiating method, which can be performed in free-breathing, might prove useful for a more thorough characterization of the disease burden in lung fibrosis, in particular of the regional blood flow in the context of new anti-fibrotic treatments available.Acknowledgements
No acknowledgement found.References
1. Block KT, Chandarana H, Milla S, et al. Towards Routine Clinical Use of Radial Stack-of-Stars 3D Gradient-Echo Sequences for Reducing Motion Sensitivity. J Korean Soc Magn Reson Med. 2014;18(2):107-119.
2. Winkelmann S, Schaeffter T, Köhler T, et al. An Optimal Radial Profile Order Based on the Golden Ratio for Time-Resolved MRI. IEEE Trans Med Imaging. 2007;26(1):68-76.
3. Grimm R, Fürst S, Dregely I, et al. Self-gated Radial MRI for Respiratory Motion Compensation on Hybrid PET/MR Systems. Med Image Comput Comput Assist Interv. 2013;16(Pt 3):17-24.
4. Bydder M, Larkman DJ, Hajnal JV. Combination of Signals From Array Coils Using Image-Based Estimation of Coil Sensitivity Profiles. Magn Reson Med. 2002;47(3):539-548.
5. Rank CM, Heußer T, Buzan MT, et al. 4D respiratory motion-compensated image reconstruction of free-breathing radial MR data with very high undersampling. Magn Reson Med. 2016; doi: 10.1002/mrm.26206
6. Yi CA, Lee KS, Han J, et al. 3-T MRI for differentiating inflammation- and fibrosis-predominant lesions of usual and nonspecific interstitial pneumonia: comparison study with pathologic correlation. AJR Am J Roentgenol. 2008;190(4):878-85.
7. Cottin V. Interstitial lung disease. Eur Respir Rev. 2013;22(127):26-32.
8. Takahashi M, Kunugi S, Terasaki Y, et al. The difference of neovascularization in early intra-alveolar fibrosis between nonspecific interstitial pneumonia and usual interstitial pneumonia. Pathol Int. 2013;63(5):237-44.
9. Walsh DA, Pearson CI. Angiogenesis in the pathogenesis of inflammatory joint and lung diseases. Arthritis Res. 2001;3(3):147-53.
Figures