0117
Free-breathing R2* Characterization of the Placenta During Normal Early Gestation Using a Multiecho 3D Stack-of-Radial Technique1Radiological Sciences, University of California Los Angeles, Los Angeles, CA, United States, 2Physics and Biology in Medicine, University of California Los Angeles, Los Angeles, CA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Siemens Healthcare, Los Angeles, CA, United States, 5Pediatrics, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 6Obstetrics and Gynecology, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 7Radiology, Weill Cornell Medical College, New York, NY, United States
Synopsis
Abnormal placental vascular development leads to ischemic-hypoxia thereby causing fetal growth restriction, preterm labor, and spontaneous abortion. Multiecho Cartesian MRI can characterize placental hypoxia by quantifying R2*, but is susceptible to motion artifacts. We have developed a new free-breathing (FB) multiecho R2* quantification technique using 3D stack-of-radial imaging (Radial). In n=16 subjects as part of an IRB-approved study, we observed an R2* range of 5 – 30s-1 at 3 T using the new FB Radial technique in the placenta during early normal gestation. Our new technique and the measured normative range of R2* may improve management of pregnancies with placental ischemic-hypoxia.
Introduction
Placental ischemic-hypoxia, a condition caused by abnormal placental vascular development, leads to negative outcomes such as fetal growth restriction, preterm labor, and spontaneous abortion1,2. Detection of placental hypoxia during early gestation can allow earlier reversal by interventions. Measurement of placental hypoxia can be performed by chorionic villous sampling, however, this is an invasive procedure with potential complications3. Alternatively, multiecho gradient echo MRI techniques can non-invasively characterize hypoxia by quantifying transverse relaxation rate (R2* = 1/T2*), which is related to tissue oxygen content4–6. However, conventional MRI techniques using Cartesian sampling are susceptible to maternal and fetal motion-induced coherent aliasing artifacts and are often performed with a breath-hold (BH)4,6. In addition, there is very limited understanding of placental R2* in both normal and abnormal early gestation. In this work, we propose and evaluate a novel free-breathing (FB) multiecho 3D stack-of-radial (FB Radial) technique for quantifying R2* in the placenta at 3 T and report results for normal early gestation.Methods
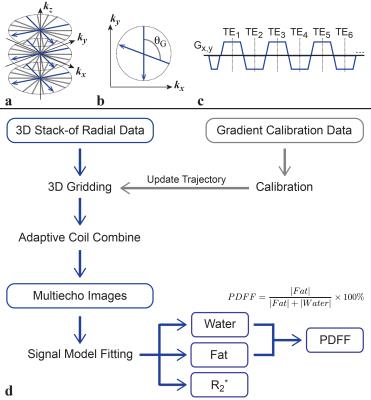
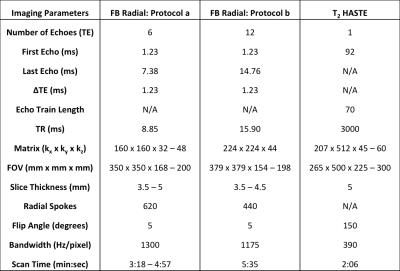
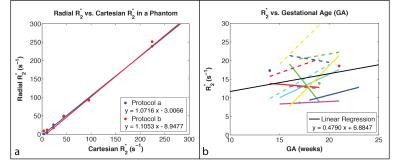
Phantom Experiments: A bipolar multi-echo RF-spoiled GRE sequence using the golden-angle-ordered(7) 3D stack-of-radial trajectory was developed (Fig. 1a-c). A phantom with test tubes containing concentrations of 30mg/mL, 10mg/mL, 6mg/mL, 3.75mg/mL, 1.875mg/mL, and 0.9375mg/mL of a superparamagnetic iron oxide agent was scanned with Cartesian (8) and Radial sequences to assess R2* with two protocols (Protocols a and b in Table 1).
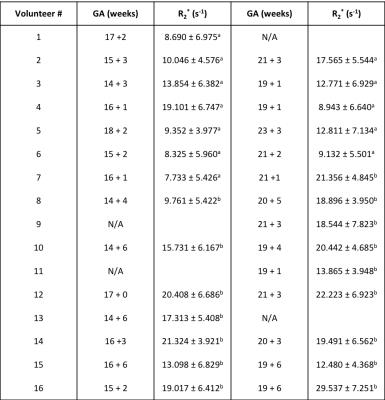
In Vivo Experiments: IRB approval and informed consent was obtained for this study. In vivo placenta scans were acquired feet-first supine in n = 16 healthy subjects during time frames of 14-18 weeks and 19-23 weeks of gestation on 3T MRI scanners (Skyra or Prisma, Siemens, Germany) using FB Radial and T2 HASTE(9). Of the 16 subjects, n=12 were scanned during both time frames, and n=4 were scanned once either during 14-18 weeks or 19-23 weeks (28 total scans) (Table 2). Protocols a and b were used in different scans to acquire images (Table 1). Initially subjects were scanned with Protocol a to get a baseline for expected R2* values. After determining that the R2* range was relatively low (5 – 30s-1), more echoes were added to improve fitting for this range. A body array coil and spine coil were used for all acquisitions.
Reconstruction: Radial reconstruction and post-processing was performed offline with MATLAB R2013b (MathWorks, Natwick, MA, USA) using gradient correction (10, 11), 3D gridding, a linear density compensation function, and adaptive coil combination(12). A 7-peak fat signal model (13) with single effective R2* per voxel was used (14–16). The Cartesian multiecho and T2 HASTE sequences were reconstructed on the scanner.
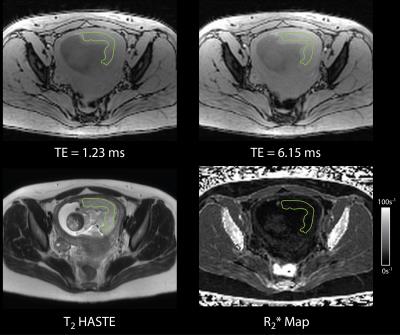
Analysis: T2 HASTE and FB Radial placental images were viewed in OsiriX 6.0 (Pixmeo, Switzerland). ROIs were drawn on the slice containing the majority of the placenta using the T2 HASTE images. The ROIs were applied to the R2* maps in anatomically corresponding regions (Fig. 2) to measure R2*. Linear correlation analysis was performed for R2* as a function of gestational age (GA) in weeks.
Results
Linear correlation analysis in the R2* phantom showed a significant correlation for Protocol a (ρ = 0.9989, P << 0.05) and Protocol b (ρ = 0.9984, P << 0.05) in mean R2* between the proposed Radial and the Cartesian techniques for the range of expected placenta R2* values (Fig. 3). This supports the use of the Radial technique for placental R2* quantification. In vivo placenta R2* maps were successfully obtained (Fig. 2). Placenta R2* values are reported in Table 2. The range of placental R2* values in early normal gestation was 5–30 s-1 at 3T. A trend towards positive correlation was observed between placental R2* and GA (Fig. 3).Discussion
Similar to our observed trend, a positive correlation between R2* and GA has also been observed in a previous study in the placenta at 1.5T for later GA of 25-30 weeks (4). With further research, we may find significant correlation between placental R2* and GA throughout gestation and characterize differences between the normal and hypoxic placenta using our FB Radial technique.Conclusion
We have demonstrated successful R2* mapping with a range of 5–30s-1 at 3T using the new FB Radial technique in the placenta during early normal gestation. Our new technique and the measured normative range of R2* may be an important foundation to further study and improve management of abnormal pregnancies with placental ischemic-hypoxia.Acknowledgements
This work acknowledges the use of the ISMRM Fat-Water Toolbox (http://ismrm.org/workshops/FatWater12/data.htm). This work was funded by the grant NICHD U01-HD087221. The authors would like to thank Irish del Rosario, Shams Rashid, and Xinran Zhong for their help with this project.References
1. Kingdom JCP, Kaufmann P. Oxygen and placental villous development: Origins of fetal hypoxia. Placenta 1997;18:613–621.
2. Parks WT. Placental hypoxia: The lesions of maternal malperfusion. Semin. Perinatol. 2015;39:9–19.
3. Rhoads GG, Jackson LG, Schlesselman SE, et al. The safety and efficacy of chorionic villus sampling for early prenatal diagnosis of cytogenetic abnormalities. N Engl J Med 1989;320:609–617.
4. Sinding M, Peters D a., Frøkjaer JB, Christiansen OB, Petersen A, Uldbjerg N, Sørensen A. Placental T2* measurements in normal pregnancies and in pregnancies complicated by fetal growth restriction. Ultrasound Obstet. Gynecol. 2015.
5. Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn. Reson. Med. 2008;60:1122–34.
6. Huen I, Morris DM, Wright C, Parker GJM, Sibley CP, Johnstone ED, Naish JH. R1 and R2 * changes in the human placenta in response to maternal oxygen challenge. Magn. Reson. Med. 2013;70:1427–33.
7. Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the golden ratio for time-resolved MRI. IEEE Trans. Med. Imaging 2007;26:68–76.
8. Zhong X, Nickel MD, Kannengiesser SAR, Dale BM, Kiefer B, Bashir MR. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn. Reson. Med. 2014;72:1353–1365.
9. Patel MR, Klufas RA, Alberico RA, Edelman RR. Half-fourier acquisition single-shot turbo spin-echo (HASTE) MR: Comparison with fast spin-echo MR in diseases of the brain. Am. J. Neuroradiol. 1997;18:1635–1640.
10. Block K, Uecker M. Simple method for adaptive gradient-delay compensation in radial MRI. Proc. ISMRM Montreal, Canada, 2011;19:2816.
11. Armstrong T, Stemmer A, Natsuaki Y, Wu HH. Free-breathing liver fat quantification using an undersampled multi-echo 3D stack-of-radial technique. In: Proc. ISMRM Suntec City, Singapore, 2016. p. 3837.
12. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn. Reson. Med. 2000;43:682–690.
13. Ren J, Dimitrov I, Sherry AD, Malloy CR. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J. Lipid Res. 2008;49:2055–2062.
14. Hernando D, Kellman P, Haldar JP, Liang Z-P. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn. Reson. Med. 2010;63:79–90.
15. ISMRM Fat Water Toolbox. 2012.
16. Gleich DF. Models and Algorithms for PageRank Sensitivity. Stanford University; 2009.
Figures