0114
Measuring temperature in brown adipose tissue using the proton chemical shift1Institute of Molecular Biosciences, University of Graz, Graz, Austria
Synopsis
Within this work an approach based on the temperature dependence of the proton resonance frequency (PRF) of water and methylene bound protons is followed to monitor in-vivo thermogenesis in the interscapular brown adipose tissue (iBAT) of mice. Measuring the change in chemical shift difference of water and fat rather than water alone, known problems of PRF-based temperature measurements such as magnetic field drift and the temperature dependence of magnetic susceptibility are circumvented. The study determines the temperature coefficient in ex-vivo iBAT tissue extracts and presents the application of the technique in measuring norepinephrine stimulated in-vivo iBAT thermogenesis.
Introduction
A feasibility study was carried out for measuring temperature in the interscapular brown adipose tissue (iBAT) by localized single voxel spectroscopy and the temperature dependence of the proton resonance frequency (PRF). iBAT consists of brown adipocytes containing numerous lipid droplets and a high concentration of mitochondria which give rise to the primary function of iBAT: generating body heat without shivering. So far, iBAT temperature has been monitored using infrared (IR) cameras1, temperature probes inserted into iBAT tissue2,3 or employing MR spectroscopy using hyperpolarized xenon dissolved in adipose tissue3. These methods have significant limitations, such as thermal isolation (fur, skin and subcutaneous fat) in a setting of external IR cameras, invasiveness and difficulties in the positioning of temperature probes and expensive hardware and complexity of hyperpolarized MR experiments. Within this study we show that iBAT tissue has excellent properties for measuring temperature based on the chemical shift difference between H2O and methylene (CH2) protons. Following this approach, common sources of errors in PRF-based temperature measurements such as magnetic field drift and the temperature dependence of magnetic susceptibility are circumvented. Based on ex-vivo iBAT tissue extracts we show that a linear coefficient describes well the temperature dependence of the chemical shift difference between H20 and CH2. Successively, the technique is used to demonstrate time-resolved in-vivo iBAT thermometry in C57BL/6 wildtype mice stimulated by norepinephrine (NE).Theory
The change in frequency of a nucleus in a molecule $$$\Delta f_{nuc}=f_{nuc}(T_2)-f_{nuc}(T_1)$$$ following a temperature change $$$\Delta T = T_2 - T_1$$$ can be approximated as4 $$$\Delta f_{nuc}=\left(-\frac{2}{3}\frac{\text{d}\chi}{\text{d}T}-\frac{\text{d}\sigma_{nuc}}{\text{d}T}\right)\Delta T + \Delta f_0$$$ with $$$\frac{\text{d}\chi}{\text{d}T}$$$ the temperature coefficient of local magnetic susceptibility, $$$\frac{\text{d}\sigma_{nuc}}{\text{d}T}$$$ the temperature coefficient of the shielding constant of that nucleus and $$$\Delta f_0$$$ a change in frequency between the two measurements due to a drift of the magnetic field or a change in the magnetic susceptibility outside the measured volume during temperature change. In iBAT tissue, following Wiedeman’s Law, protons bound to water and methylene share a common local magnetic susceptibility and therefore temperature coefficient $$$\frac{\text{d}\chi}{\text{d}T}$$$. In addition, the macroscopic shift in frequency $$$\Delta f_0$$$ affects both nuclei in the same way. Therefore, the change in chemical shift difference of water and fat (-CH2) protons at two different temperatures $$$\delta_{W-F}=\Delta f_W - \Delta f_F$$$ is $$\delta_{W-F}=\left(-\frac{\text{d}\sigma_W}{\text{d}T}+\frac{\text{d}\sigma_F}{\text{d}T}\right)\Delta T= \frac{\text{d}\sigma_{W-F}}{\text{d}T}\Delta T$$ with $$$\frac{\text{d}\sigma_{W-F}}{\text{d}T}$$$ the temperature coefficient of the chemical shift difference between H20 and CH2 protons. With known temperature coefficient the temperature can be measured: $$\Delta T= \frac{\delta_{W-F}}{\frac{\text{d}\sigma_{W-F}}{\text{d}T}}$$Methods
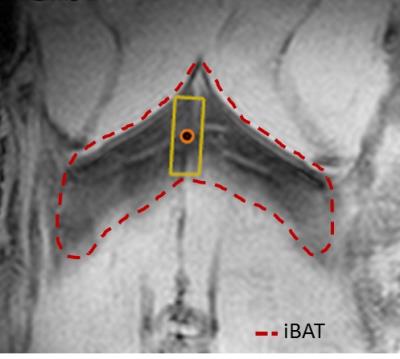
Measurements were carried out at 7 T using a 20 mm surface coil. Ex-vivo temperature coefficient measurement: fresh iBAT tissue extracts (n=6) were put into a 500 µl syringe tube and placed parallel to the main magnetic field. Sample temperature [26,29,32,35,38,41]°C was controlled by a water perfused silicon pad wrapped around the syringe and measured within the sample by a thermistor probe. Single voxel 1H spectra were acquired at each temperature step employing a PRESS protocol: TR/TE = 2500/18 ms, BW = 3500 Hz, voxel size 2.5x2.5x2.5 mm, 1 average. Water and CH2 center frequencies were determined using jMRUI and AMARES. In-vivo study: Room temperature (22±2 °C) acclimated male C57BL/6 mice (n=3) were measured at an age of 18 weeks. The animals were anesthetized with an i.p. injection of 60 mg/kg of pentobarbital with a maintenance dose of ¼ every 40 min. The mice were placed in supine position with the surface coil placed below the iBAT. The animal bed was heated to 30 °C. Rectal temperature was continuously monitored. The spectroscopic voxel was positioned away from large vessels (see Fig. 3). The same PRESS protocol and analysis as for the ex-vivo study was used but: voxel volume ~ 4 µl, averages = 32, respiratory triggered with outer volume suppression. Every 5 minutes a spectrum was recorded. After 3 baseline measurements, iBAT thermogenesis was stimulated by an i.p. injection of 1mg/kg norepinephrine and monitored for 2 hours.Results & Discussion
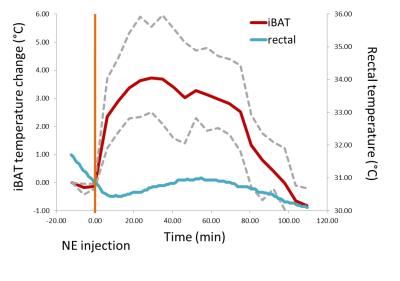
From ex-vivo measurements presented in Fig. 1&2, the iBAT temperature coefficient was determined by linear regression to be $$$\frac{\text{d}\sigma_{W-F}}{\text{d}T}=-0.0106 \pm1.5\cdot10^{-4}\frac{\text{ppm}}{\text{°C}}$$$ (±SE). Using this coefficient, in-vivo iBAT temperature was measured in a setting of NE-stimulated iBAT thermogenesis (Fig. 4). The average iBAT temperature increase of 3.72 °C is in good agreement to measurements with invasive temperature probes2,3. In addition, time-resolved thermometry shows that the peak in iBAT temperature is followed by an increase of rectal temperature which is again consistent with other studies2,3. Summarizing, the temperature dependent chemical shift difference of H20 and CH2 measured from single voxel 1H spectra offers a reliable yet easy to apply method for in-vivo thermometry in brown adipose tissue.Acknowledgements
Grant 12CVD04 from the Leducq Foundation, F30 SFB-LIPOTOX, Wittgenstein Award Z136 and W901-B05DK (Molecular Enyzmology) funded by the Austrian Science Foundation (FWF).References
[1] Crane J.D. et al. in Mol Metab. 2014; 3(4):490-494. [2] Khanna A., Branca R.T. in Magn Reson Med. 2012; 68(4):1285-1290. [3] Branca R.T. et al. PNAS 2014; 111(50): 18001-18006. [4] Stollberger R. et al. in JMRI 1997; 8:188-196.Figures
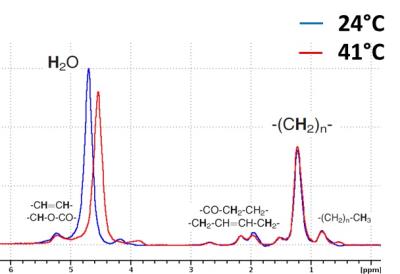
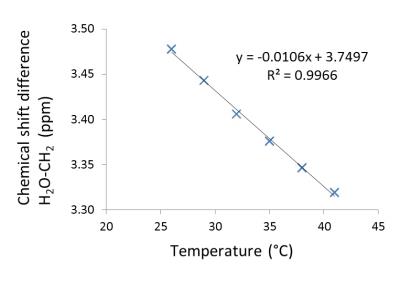
Figure 2: Ex-vivo measured chemical shift difference between H2O and CH2 protons in iBAT tissue extracts as a function of temperature.