0112
Accelerated Segmented Diffusion-Weighted Prostate Imaging for Higher Resolution, Higher Geometric Fidelity, and Multi-b Perfusion QuantificationPelin Aksit Ciris1, Jr-yuan George Chiou2, Daniel Glazer2, Shelley Hualei Zhang2, Tzu-Cheng Chao3, Bruno Madore2, and Stephan Ernst Maier2,4
1Department of Biomedical Engineering, Akdeniz University, Antalya, Turkey, 2Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States, 3Department of Computer Science, National Cheng Kung University, Tainan City, Taiwan, 4University of Gothenburg, Gothenburg, Sweden
Synopsis
An accelerated multi-shot diffusion imaging scheme was developed for prostate imaging. Its robustness to regularization was evaluated. Two-fold improvement in spatial resolution and over three-fold improvement in geometric fidelity were obtained as compared to single-shot EPI, in twenty-five prostate cancer patients. In contrast to the standard protocol, which involves separate scans with high (b=1400 and 0 s/mm2) and intermediate (b=500 and 0 s/mm2) diffusion weighting, the proposed accelerated protocol extended b-factor coverage to yield an additional 8 b-factors and enabled multi-b perfusion quantification in half the scan time (5 min 43 s vs. 11 min 48 s).
Purpose
To improve the speed, spatial-resolution and geometric-fidelity of diffusion-weighted-imaging (DWI) in the prostate, evaluate the effect of regularization, and estimate perfusion from multiple b-factors acquired in the same session.Introduction
Prostate cancer (PCa) is very common and affects approximately one man in every six. DWI is an essential component of PCa diagnosis and staging [1, 2], however, suffers from low resolution and geometric distortions. Although multi-coil acceleration can reduce distortions, this is not practical for prostate imaging with a single, endo-rectal RF coil. A recent accelerated multi-shot acquisition method [3] exploits the sparsity of diffusion-encoded data in the x-y-kb-kd space (where kb and kd are Fourier-transform duals of the b-factor and the diffusion direction, respectively), to displace aliasing artefacts toward underused regions of the kb-kd plane, and recover non-aliased signals, at potentially no cost in scan time. This acceleration scheme is fully compatible with a single coil configuration and was used to acquire many b-factors at high resolution with good geometrical fidelity in a short scan time in the prostate. The influence of regularization was evaluated and perfusion parameters were estimated.Methods
Twenty-five patients undergoing PCa staging participated in this IRB-approved study (ages: 63±8 years). Imaging was performed at 3 Tesla (MR750w system, GE Healthcare) using an endo-rectal coil (Medrad). T2-weighted imaging was followed by conventional DWI (DWIC): 1.9mm x 1.9mm, 22 4mm slices, 96x77 matrix, 71% partial Fourier, 180x144mm FOV, 167/1.3 kHz BW (read/phase), b-factors (averages): 0 (1) and 500 (8) s/mm2 and TE/TR 64/6350ms, or 1400 (16) s/mm2 and TE/TR 79/7550ms. For high quality DWI at diagnostically relevant b-factors (500 and 1400 s/mm2) and a range of b-factors suitable for perfusion analysis, a protocol with variable density b-factor sampling was designed for accelerated DWI (DWIA): 1.4mm x 1.4mm, 22 4mm slices, 128x128 matrix, 180x180mm FOV, 167/4.4 kHz BW (read/phase), b-factors (averages): 12.5 (4), 25 (1), 50 (1), 75 (1), 100 (1), 150 (1), 200 (1), 300 (1), 400 (1), 500 (4) and 1400 (8) s/mm2 and TE/TR 78/5050ms. A crusher gradient-free design was employed with a minimum b-factor of 12.5 s/mm2 for adequate alternate signal crushing, to ensure correct diffusion encoding at very low b-factors. For multi-shot DWIA, the segmentation factor equaled the acceleration factor (R=4); thus for each diffusion encoding direction and each b-factor 32 echoes were sampled. Data were reconstructed as introduced in [3], using the magnitude and phase data from a concurrently acquired low-resolution 2D navigator echo (matrix=32x32, TE=128.4ms) for regularization and motion correction, respectively, with regularization strengths λ of 0.001, 0.002, 0.005, 0.01, 0.05, and 0.1. The effect of regularization on agreement of conventional and accelerated ADC was evaluated using linear regression analysis. Tumors and normal peripheral zone (PZ) and central gland (CG) were delineated on DWIC (b=500 and 1400 s/mm2) and direction-averaged DWIA. ADC maps were calculated from non-linear least-square fits with mono-exponential functions, to S/S0 = exp(-bD) using b-factors up to b=500 or b=1400 s/mm2 weighted by the number of averages. Perfusion-free diffusion coefficients (D) and perfusion fractions (f) were calculated in each ROI similarly from fits to S/ S0 = exp(-bD) using b=150 through 500 s/mm2, S/ S0’ = exp(-bD’) using b=0 through b=150 s/mm2, and f = (S0’ - S0)/S0’.Results
Eighteen patients had lesions; nine had a Prostate Imaging Reporting and Data System (PI-RADS) score of 5, nine PI-RADS 4, and one PI-RADS 2. DWIA produced diagnostic quality high-resolution images and ADC maps (Fig.1). Regularization with λ = 0.01 optimized the agreement of conventional and accelerated ADCs (Figs.2 and 3). Geometric-fidelity improved by a factor of 3.2 ± 1.1 over DWIC (Fig.4). Multi-b perfusion was estimated as depicted in Fig. 5. Across all patients, f was slightly higher in tumors than in normal PZ (P = 0.12) and significantly higher in normal CG than in normal PZ (P = 0.00013). D differed significantly between tumors and normal PZ as well as normal CG (P < 10-8).Discussion
Acquiring 2 single b-factors and b0 took nearly 12 min with single-shot EPI. In contrast, our acceleration scheme enabled acquisition of 11 b-factors at two-fold the resolution in under 6 min, with over three-fold geometric-fidelity improvement (nearly at the theoretically-expected 3.33-fold). These improvements were achieved with a single-channel coil and further improvements in DWIA may be possible with the addition of multi-channel coils. Perfusion parameters highlighted significant differences between tumors and normal tissues, offering great potential for improved prostate lesion characterization [4-6].Conclusion
Accelerated prostate DWI with high spatial-resolution, geometric-fidelity, and perfusion quantification using multiple b-factors was feasible while maintaining good SNR and reasonable scan times.Acknowledgements
TUBITAK 116C024, NIH R01CA160902, R01EB010195 and R01CA149342.References
1. Tempany, C.M., et al., Multimodal imaging for improved diagnosis and treatment of cancers. Cancer, 2014. doi: 10.1002/cncr.29012. 2. Hegde, J.V., et al., Multiparametric MRI of prostate cancer: an update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. Journal of Magnetic Resonance Imaging, 2013. 37(5): p. 1035-54. 3. Madore, B., et al., Accelerated multi-shot diffusion imaging. Magn Reson Med, 2014. 72(2): p. 324-36. 4. Dopfert, J., et al., Investigation of prostate cancer using diffusion-weighted intravoxel incoherent motion imaging. Magn Reson Imaging, 2011. 29(8): p. 1053-8. 5. Shinmoto, H., et al., Biexponential apparent diffusion coefficients in prostate cancer. Magn Reson Imaging, 2009. 27(3): p. 355-9. 6. Zhang, Y.D., et al., The histogram analysis of diffusion-weighted intravoxel incoherent motion (IVIM) imaging for differentiating the gleason grade of prostate cancer. Eur Radiol, 2015. 25(4): p. 994-1004.Figures
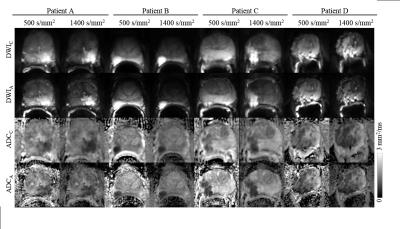
Conventional and accelerated diffusion weighted
images at b=500 s/mm2 and b=1400 s/mm2, and ADC maps from b=500 s/mm2 and
b=1400 s/mm2 (from four sample patients).
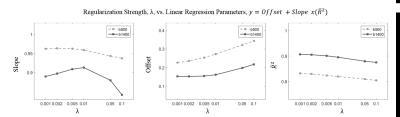
Effect of regularization on agreement of
conventional ADC (from b=0 and b=500 or 1400 s/mm2) and accelerated ADC (from
b=12.5 and 500 or 1400 s/mm2). Changes in the (a) Slope (b) Offset, and (c)
Adjusted coefficient of determination of the linear regression analysis are
shown as a function of the regularization parameter λ.
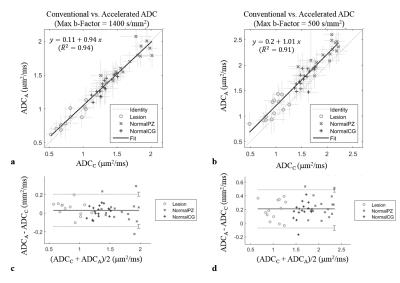
Agreement between conventional and accelerated
ADC values for all patients and ROIs (o: lesions, x: normal CGs, +: normal PZ)
(left: b=1400, right: b=500; top: Scatter plots of conventional vs. accelerated
ADCs overlaid with the identity line, regression lines and adjusted
coefficients of determination; bottom: Bland-Altman plots (-- mean difference, …
95% CI at +/-1.96 SD).
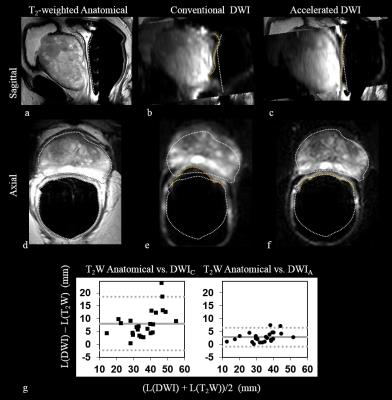
(a-f)
T2-weighted anatomical, conventional and accelerated b=0 images from DWI. The prostate-rectal wall boundary (white on
T2-weighted anatomical; yellow on diffusion-weighted conventional and
accelerated images), is severely distorted in conventional DWI. Accelerated DWI
improves geometric fidelity. (g) Bland-Altman plots of the extent of geometric
distortions in the b=0 image of the
diffusion-weighted acquisition relative to T2W anatomical imaging, for all
subjects (L= size of the prostate in a given acquisition, in the
anterior-posterior phase-encoding direction, in mm.) (-- mean difference and …
95% CI at +/-1.96 SD). DWIA improves geometric fidelity by a factor
of 3.2 +/- 1.1.
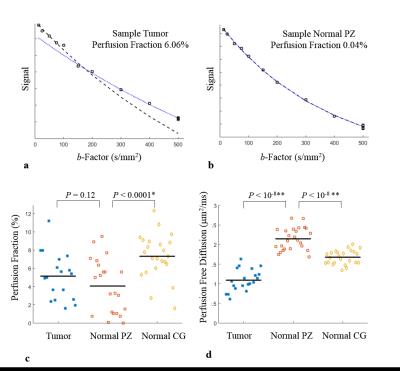
(a) Signal vs. b-factor and mono-exponential fits
to b ≤150 s/mm2 (--) and b ≥ 150 s/mm2 (…), shown in a sample (a)
tumor (perfusion fraction: 6.06%), and (b) normal PZ (perfusion fraction: 0.04%).
Perfusion fraction (c) and perfusion free diffusion coefficient (d) estimates
from all tumor (blue), normal PZ (red) and normal CG (yellow) ROIs; perfusion
fraction was slightly higher in tumors than in normal PZ (P = 0.12), and significantly higher in normal CG than in normal PZ
(P = 0.00013). Perfusion free
diffusion differed significantly between tumors and normal PZ or CG (P < 10-8).