0110
Optogenetically-evoked somatosensory inputs enhance sound processing in the auditory system1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, Hong Kong, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, Hong Kong
Synopsis
Brain-wide cross-modal interactions are important for building an accurate perception of the external world. Yet, whether and how somatosensory inputs influence the auditory processing remains unclear. Our recent study showed that
Purpose
The brain integrates and distributes information from different sensory modalities to form a representation of the environment and facilitate behavioral responses. Comparative to the visual-auditory and somatosensory-visual systems1-3, interactions within the somatosensory-auditory system are not well understood. Recent studies revealed that, in addition to auditory cortex (AC), lower structures within the central auditory ascending pathway also receives numerous long-range projections from somatosensory cortices4,5. Furthermore, a study showed convergence of auditory and somatosensory inputs in AC using fMRI6. Together, these studies indicate functional relevance of the auditory-somatosensory integration was mediated by long-range projections. Optogenetic fMRI (ofMRI) is a potent tool to monitor the global effects of modulating local neuronal population7. Our recent ofMRI study demonstrated that responses induced by low frequency optogenetic stimulation of the ventral posteromedial thalamus (VPM) excitatory thalamocortical neurons are not limited to monosynaptic thalamo-cortical projections from VPM to somatosensory cortices, as activity propagates brain-wide to remote sensory cortices including AC8. This observation suggests that such long-range low frequency activity propagation may underlie cross-modal inputs from the somatosensory to auditory system. In this study, we aimed to determine whether brain-wide propagation of low frequency activity from the somatosensory system influences sound processing in the auditory system by combining auditory and optogenetic fMRI9.Methods
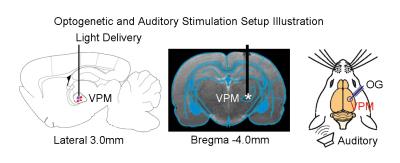
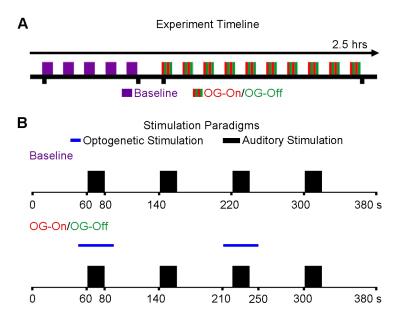
AAV5-CaMKIIα-ChR2(H134R)-mCherry was injected to the VPM of adult Sprague-Dawley rats (n=6, 200-250g, male). After four weeks, an optical fiber cannula (d=450μm) was implanted at the injection site (Figure 1). All MRI experiments were performed under 1.0% isoflurane. Monaural (left) noise stimulation (sound pressure level=85dB) was presented in a block design paradigm (20s on and 60s off, 4 blocks). To investigate whether auditory processing was influenced by low frequency optogenetic stimulation, 1Hz blue light (10% duty cycle, 40mW/mm2) was presented 10s before to 10s after every even sound-on period. (Figure 2). All fMRI data was acquired at 7T using GE-EPI (FOV=32×32mm, matrix=64×64, α=56°, TE/TR=20/1000ms, sixteen 1mm contiguous slices). Data were preprocessed and averaged by blocks before standard GLM analysis was applied to identify significant BOLD responses.Results
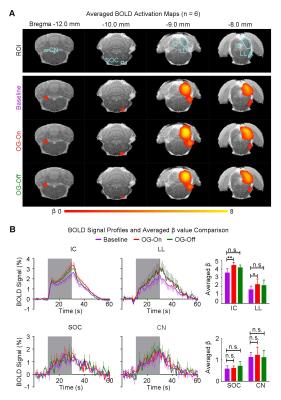
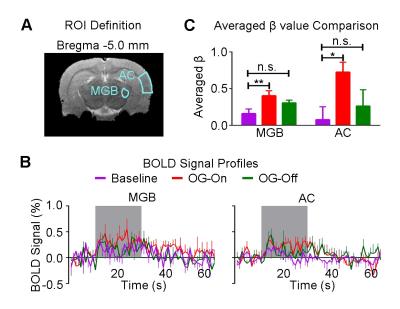
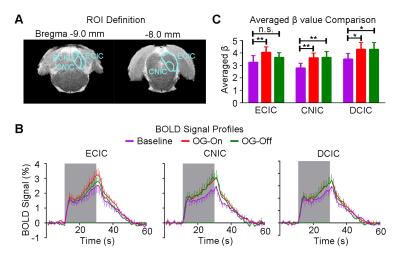
Figure 3 shows the auditory-evoked BOLD responses to noise stimulation in the inferior colliculus (IC), lateral lemniscus (LL), superior olivary complex (SOC) and cochlear nucleus (CN) before (Baseline), during (OG-On) and after (OG-Off) 1Hz optogenetic stimulation (P<0.05, FWE correction). During optogenetic stimulation, auditory-evoked responses in IC and LL were increased (P<0.05, paired t-test). Figure 4 presents the responses in MGB and AC was enhanced by optogenetic stimulation (P<0.05, paired t-test). Figure 5 shows the BOLD signal profiles and averaged β comparison in different IC subregions, including external cortex of the IC (ECIC), central nucleus of the IC (CNIC) and dorsal cortex of the IC (DCIC). Responses in all three subregions were increased during the optogenetic stimulation. After the cessation of stimulation, responses remained elevated in CNIC and DCIC (P<0.05, paired t-test).Discussion and Conclusion
Our fMRI results directly demonstrated that low frequency optogenetic stimulation of the somatosensory thalamic structure - VPM enhanced the auditory responses in AC, MGB, IC and LL, but not the lower structures of the central auditory pathway (i.e., SOC and CN). The recent ofMRI study revealed that low frequency optogenetic excitation of VPM can induce brain-wide BOLD activation in cortical regions, including the auditory cortex8. Our present ofMRI study clearly demonstrated that such low frequency optogenetic VPM modulation can enhance auditory BOLD response in AC, auditory midbrain - IC and LL, and thalamus - MGB. Our findings suggested that somatosensory-auditory interactions were underpinned by brain-wide low frequency cortical activity. Note that it is now well-known that IC, LL and MGB responses are modulated by AC through the auditory descending pathway10,11, or through indirect and direct projections from somatosensory cortices12. More interestingly, the profiles in subregions of the IC (i.e. ECIC, CNIC and DCIC) showed different trends in response to the optogenetic modulation (Figure 5), suggesting the auditory response enhancement across IC subregions by low frequency VPM stimulation might occur via different mechanisms. Particularly, enhancement in ECIC was not maintained after optogenetic stimulation, similar to AC. Future studies will explore the mechanism of such enhancement effect with the aid of electrophysiological recording, and examine the influence of low frequency activity propagation on more advanced aspects of sound processing, such as tonotopy and vocalization, in the auditory system. In conclusion, brainwide cortical propagation of long-range low frequency inputs from somatosensory system enhances sound processing in the auditory system, including AC, MGB, IC and LL. Our study highlights that large view fMRI in combination with sensory stimulation and optogenetic modulation can offer a highly specific imaging tool in dissecting brain-wide networks and functions.Acknowledgements
No acknowledgement found.References
1. F. Crevecoeur, D. P. Munoz, and S. H. Scott, "Dynamic Multisensory Integration: Somatosensory Speed Trumps Visual Accuracy during Feedback Control," J Neurosci, vol. 36, pp. 8598-611, Aug 17 2016.
2. K. Sieben, B. Roder, and I. L. Hanganu-Opatz, "Oscillatory entrainment of primary somatosensory cortex encodes visual control of tactile processing," J Neurosci, vol. 33, pp. 5736-49, Mar 27 2013.
3. T. M. Van Vleet and L. C. Robertson, "Cross-modal interactions in time and space: auditory influence on visual attention in hemispatial neglect," Journal of cognitive neuroscience, vol. 18, pp. 1368-1379, 2006.
4. J. U. Henschke, T. Noesselt, H. Scheich, and E. Budinger, "Possible anatomical pathways for short-latency multisensory integration processes in primary sensory cortices," Brain Struct Funct, vol. 220, pp. 955-77, Mar 2015.
5. K. G. Gruters and J. M. Groh, "Sounds and beyond: multisensory and other non-auditory signals in the inferior colliculus," Front Neural Circuits, vol. 6, p. 96, 2012.
6. J. J. Foxe, G. R. Wylie, A. Martinez, C. E. Schroeder, D. C. Javitt, D. Guilfoyle, et al., "Auditory-somatosensory multisensory processing in auditory association cortex: an fMRI study," Journal of Neurophysiology, vol. 88, pp. 540-543, 2002.
7. J. H. Lee, R. Durand, V. Gradinaru, F. Zhang, I. Goshen, D. S. Kim, et al., "Global and local fMRI signals driven by neurons defined optogenetically by type and wiring," Nature, vol. 465, pp. 788-92, Jun 10 2010.
8. A. T. L. Leong, R. W. Chan, P. P. Gao, Y. S. Chan, K. K. Tsia, W. H. Yung, et al., "Long-range projections coordinate distributed brain-wide neural activity with a specific spatiotemporal profile," Proc Natl Acad Sci U S A, 2016 (in press).
9. P. P. Gao, R. W. Chan, A. T. L. Leong, and E. X. Wu, "Combined auditory and optogenetic fMRI for investigation of visual cortical descending modulation of auditory midbrain processing," Proceedings of the 24rd Annual Meeting of ISMRM, Singapore, p. 0481, 2016.
10. P. P. Gao, J. W. Zhang, S.-J. Fan, D. H. Sanes, and E. X. Wu, "Auditory midbrain processing is differentially modulated by auditory and visual cortices: An auditory fMRI study," NeuroImage, vol. 123, pp. 22-32, 2015.
11. J. A. Winer, M. L. Chernock, D. T. Larue, and S. W. Cheung, "Descending projections to the inferior colliculus from the posterior thalamus and the auditory cortex in rat, cat, and monkey," Hearing research, vol. 168, pp. 181-195, 2002.
12. S. Shore, "Auditory/somatosensory interactions," 2009.
Figures