0109
Functional MRI evaluation of a novel approach to neuromodulation: Targeted delivery of GABA via focused ultrasound-mediated disruption of the blood-brain barrier1Brigham and Women's Hospital, Boston, MA, United States, 2Department of Neurobiology, Harvard Medical School, Boston, MA, United States
Synopsis
Here we present a novel approach to non-invasive neuromodulation that affects neuronal activity by delivering neurotransmitter chemicals to targeted areas of the brain. This is achieved by using focused ultrasound to transiently open the blood-brain barrier in a targeted brain region such that a systemically injected neuroactive chemical such as GABA or glutamate will leak out of the vessels and into the brain parenchyma only at the intended site. We demonstrate the proof of concept in a rodent model by delivering GABA to the somatosensory cortex to suppress activation from hindpaw stimulation.
Introduction
Neuromodulation refers to the selective enhancement or suppression of neuronal activity in targeted brain regions and represents an important tool for both basic science research and clinical applications1. A variety of neuromodulation techniques have been developed, such as optogenetics or transcranial magnetic stimulation, and their effects on brain function can be assessed using functional MRI2.
Here we present a novel approach to non-invasive neuromodulation that affects neuronal activity by delivering neurotransmitter chemicals to targeted areas of the brain3. This is achieved by using focused ultrasound (FUS) to transiently open the blood-brain barrier (BBB) in a targeted brain region4 such that a systemically injected neuroactive chemical such as GABA or glutamate will leak out of the vessels and into the brain parenchyma only at the intended site. This work shows initial results demonstrating the proof of concept in a rodent model using delivered GABA to modulate neuronal activity and fMRI to measure the effects.
Methods
Functional activation was assessed in six Sprague-Dawley rats undergoing hindpaw stimulation using a 2x2 factorial design with conditions of BBB Closed vs BBB Open and No GABA Injected vs GABA Injected.
Stimulation: Bilateral hindpaw electrical stimulation (4 mA, 0.3 ms duration, 2 Hz) for activation of the somatosensory network. Rats were under light anesthetic sedation using Dexdormitor (0.4 mg/kg bolus followed by 0.4 mg/kg/hr continuous subcutaneous infusion)5 with respiratory rate monitored throughout.
BBB opening: The somatosenory cortex (S1HL) in the right hemisphere only was targeted for BBB disruption. Microbubbles were injected (Optison, 200 μl/kg) and 690 kHz transcranial FUS was applied using 10 ms bursts at 1 Hz for 120 seconds. The extent and location of BBB opening were evaluated after the fMRI sessions with T1-weighted Gadolinium contrast imaging.
GABA delivery: Systemic tail vein bolus injection of 50 mg/kg of GABA was delivered immediately before the second fMRI session.
fMRI: Rats underwent four total fMRI sessions, two on a day without BBB opening and two on a day with BBB opening. Images were acquired on a Bruker 7T scanner with a single shot EPI sequence (TR = 1.5 s, TE = 18 ms, 18 slices, 0.5 x 0.5 x 1.0 mm resolution, 300 images). Stimulation was performed in a 40 s OFF, 20 s ON block design over 7.5 minutes. Data were processed using SPM12 with realignment, coregistration to a T2w anatomical image, normalization to a common space, and spatial smoothing. A general linear model framework was used to detect regions of significant stimulus-correlated activation.
Results
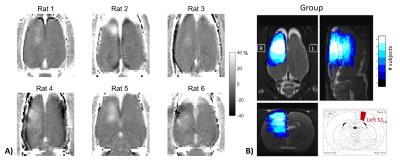
BBB opening results are shown in Figure 1. The images in Figure 1A show the percent change in signal magnitude for the Gadolinium contrast imaging done on each rat (coronal slice through the cortex). Hyperintense regions are indicative of BBB disruption. Figure 6B summarizes BBB opening for all rats on the same anatomical image, with the S1HL region shown from the Paxinos-Watson atlas for reference6. FUS targeting of the S1HL region was accurate enough such that BBB disruption was confined to the right hemisphere in all six rats and covered the S1HL region in five out of six rats.
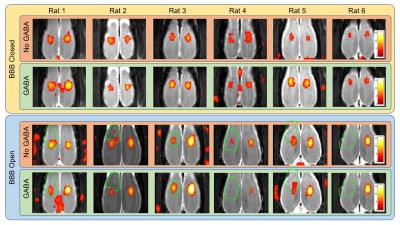
Figure 2 shows the fMRI activation results for each individual rat over the four conditions. The green outlines indicate the region of BBB opening. Consistent bilateral activation in the S1HL region is seen for both GABA and No GABA conditions when the BBB is closed.
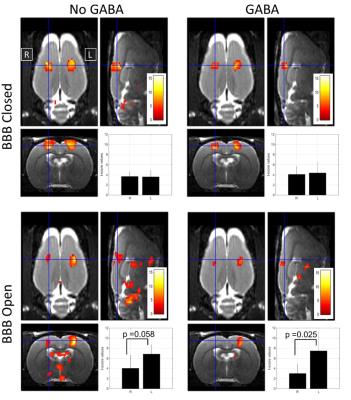
Figure 3 shows the group activation results over the six rats for the four conditions. The bar plots display mean t-score values over an ROI that covered the left or right S1HL region (mean and standard deviation over rats). The differences between activation in left vs right hemispheres were assessed using a two-tailed t-test. The t-score metrics indicate a trend in decreased activation for BBB disruption alone (No GABA injection), as also observed by Chu et al7, and a significant decrease in activation for BBB disruption plus GABA injection.
Discussion & Conclusions
These initial results are a promising indicator that FUS can be used to disrupt the BBB to allow delivery of neurotransmitter chemicals that affect local brain function. Immediate future work will focus on titrating the FUS parameters and GABA dosage levels to minimize effects due to BBB disruption alone and maximize effects due to the combination of BBB opening and GABA delivery. Other future work will consider delivery of different neurotransmitter chemicals (e.g. glutamate) and targeting of different brain region (e.g. thalamus).Acknowledgements
NIH grants R25CA089017-13 and P01CA174645, Focused Ultrasound Surgery Foundation 2016 Global Internship Program, and The Brigham Research Institute.References
1. Luan, S. et al., 2014. Neuromodulation?: present and emerging methods. Fronteirs in Neuroengineering, 7(July), pp.1–9.
2. Liang, Z. et al., 2015. Mapping the functional network of medial prefrontal cortex by combining optogenetics and fMRI in awake rats. NeuroImage, 117, pp.114–123.
3. McDannold, N. et al., 2015. Targeted, noninvasive blockade of cortical neuronal activity. Scientific reports, 5, p.16253.
4. Hynynen, K. et al., 2001. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology, 220(3), pp.640–6.
5. Adamczak, J.M. et al., 2010. High field BOLD response to forepaw stimulation in the mouse. NeuroImage, 51(2), pp.704–712.
6. Paxinos, G., and Watson, C. 1997. The Rat Brain in Stereotaxic Coordinates. Third Edition. Academic Press, San Diego, CA.
7. Chu, P. et al., 2015. Neuromodulation accompanying focused ultrasound-induced blood-brain barrier opening. Scientific Reports, 5:15477.
Figures