0107
Chemo-fMRI: a DREADD-based approach to unravel the brainwide substrates of neuromodulation1Biology Department, University of Pisa, Pisa, Italy, 2Functional Neuroimaging Laboratory, Center for Neuroscience and Cognitive Systems, Istituto Italiano di Tecnologia, Rovereto, Italy, 3Neuroscience and Brain Technologies Department, Istituto Italiano di Tecnologia, Genova, Italy
Synopsis
Notable examples of the combined use of optogenetics an fMRI (i.e. “opto-fMRI”) have been recently published, revealing the possibility to map the brainwide substrates modulated by focal neuronal population. However, opto-fMRI is complicated by the use of invasive cranial implants, and the need to control the insidious contribution of heat-induced hemodynamic–responses. Here we show that “chemo-fMRI”, e.g. the combined us of DREADD-based chemogenetics and fMRI, permits to overcome these limitations, by enabling non invasive brainwide mapping of tonically-stimulated neuromodulatory systems. Chemo-fMRI mapping of serotonin-producing neurons is described as an illustrative example of the power of this novel investigational approach.
Introduction
The advent of cell-manipulation methods has made possible to causally link the activity of focal neuronal populations with specific circuital and behavioural outputs. A few notable studies have expanded this paradigm to the investigation of brainwide patterns of brain function via the combined use of fMRI and optogenetics, an approach termed “opto-fMRI”[1, 2]. While versatile and adaptable to the investigation of diverse neuronal population, opto-fMRI approaches are however hampered by two major limitations: (a) they are intrinsically invasive, requiring the implant of cranial probes (b) and they entail a tight control of noxious heat-induced artefacts, an effect that can produce deceiving circuit-like haemodynamic responses [3, 4].
The use of chemogenetic approaches to neuronal modulation such as Designed Receptors Exclusively Activated by Designed Drugs (DREADDs) [5] can potentially overcome the limitations of opto-fMRI when a tonic control of neuronal activity is required. DREADD receptors are human muscarinic receptors that exhibit high specificity for the brain penetrant and otherwise pharmacological inert compound Clozapine N-Oxide (CNO), and display negligible affinity for their native ligand acetylcholine[6]. This property can be exploited to permit remote manipulation of neuronal population in living mice during fMRI acquisitions, via the systemic administration of CNO. Towards this goal, here we describe the combined used of chemogenetics and fMRI (which we term “chemo-fMRI”) to map the brainwide targets focal neural manipulations. To provide a proof of principle demonstration of chemo-fMRI, we show its use in the investigation of the brainwide substrates modulated by midbrain serotonin (5HT)-producing neurons, an archetypical neural systems characterized by restricted spatial localization but with broad modulatory properties.
Methods
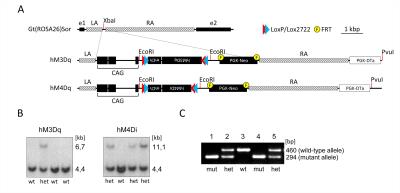
All experiments were carried out in accordance with Italian regulations governing animal welfare and protection. To induce pan-5HT neuronal targeting, we generated stable conditional knock-in mouse models carrying floxed versions of excitatory hM3Dq and inhibitory hM4Di DREADD receptors (Fig. 1.) The mouse lines were then crossed with PET1-cre to enable selective expression in 5-HT producing neurons. Cell-type specificity and functional activity of hM3Dq and hM4Di were probed using immunohistochemical staining, and whole cell patch-clamp electrophysiology, respectively [7]. fMRI studies were carried out as previously described [8]. Briefly, mice were anaesthetised with isoflurane, intubated and artificially ventilated. Image acquisition was performed under 0,8% halothane anaesthesia [9]. fMRI data were acquired at 7 T using a FLASH sequence (TR = 288 ms, TE = 3.1 ms, α=30°; 180 x 180 x 600 µm resolution, dt = 60 s, Nr = 60). Images were sensitized to reflect alterations in rCBV [8] upon injection of 5 µl/g of blood-pool contrast agent (Molday Ion, Biopal). Each experimental group consisted of at least 6 subjects. CNO was injected i.v. in hM3Dq-bearing and double-floxed inversed (DIO) controls animals at a dose of 0.5 mg/kg. Individual fMRI subject response amplitude maps were calculated within the framework of the general linear model using a boxcar model function capturing the sustained response produced by CNO.Results and discussion
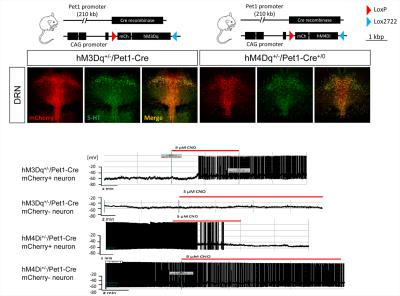
Immunohistochemical mapping confirmed the regional selectivity of hM3Dq and hM3Di expression in 5HT-producing raphe nuclei (Fig.2). Whole-cell patch clamp electrophysiology revealed selective DREADD-induced functional activity in 5-HT producing neurons, with evidence of hyperpolarization (hM3Di) or depolarization (hM3Dq) upon exposure to CNO (Fig. 2).
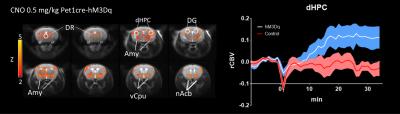
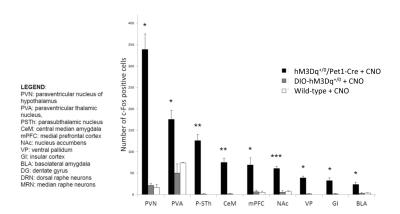
In vivo dose-response curves of CNO in control animals revealed unspecific functional alterations in several brain regions at doses higher than 1 mg/kg, but not at 0.5 mg/kg. These results suggest that DREADD-based chemo-fMRI mapping need to be quantified at low CNO doses, and contrasted with control group of subjects lacking DREADD receptors. Such an approach was employed to map of the effect of pan-5HT stimulation via CNO-induced activation of hM3Dq receptors (Fig.3). Chemo-fMRI activation of 5-HT neurons resulted in robust regional fMRI activation encompassing brain substrates involved in emotional responses (raphe nucleus, hippocampus, amygdala), plus a previously unreported focal activation of core mesolimbic dopamine reward-systems. Importantly, an analogous pattern of functional activation was obtained using c-Fos immunohistochemical mapping upon CNO administration in freely-moving animals, hence corroborating a neuronal origin of the changes mapped, and arguing against a confounding contribution of anaesthesia (Fig. 4).
Conclusion
Our data serve as a first proof-of-principle demonstration of the possibility to combine fMRI and chemogenetics to unravel the large-scale substrates modulated by focal neuronal stimulations. We show that the ensuing chemo-fMRI responses exhibit exquisite neuronal specificity, are sustained, and can be employed to obtain to non-invasive map regional manipulations in the living mouse brain. The approach permits to causally probe the role of focal neural populations in affecting large-scale neuromodulatory states and brainwide network activity, thus effectively complementing current opto-fMRI paradigms.Acknowledgements
No acknowledgement found.References
[1] Lee, J. H.; Durand, R.; Gradinaru, V.; Zhang, F.; Goshen, I.; Kim, D. S.; Fenno, L. E.; Ramakrishnan, C.; Deisseroth, K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring, Nature (2010) 465, 788-792.
[2] Ferenczi, E. A.; Zalocusky, K. A.; Liston, C.; Grosenick, L.; Warden, M. R.; Amatya, D.; Katovich, K.; Mehta, H.; Patenaude, B.; Ramakrishnan, C. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior, Science (2016) 351, aac9698.
[3] Schmid, F.; Wachsmuth, L.; Albers, F.; Schwalm, M.; Stroh, A.; Faber, C. True and apparent optogenetic BOLD fMRI signals, Magnetic resonance in medicine (2016).
[4] Christie, I. N.; Wells, J. A.; Southern, P.; Marina, N.; Kasparov, S.; Gourine, A. V.; Lythgoe, M. F. fMRI response to blue light delivery in the na+»ve brain: Implications for combined optogenetic fMRI studies, NeuroImage (2013) 66, 634-641.
[5] Roth, Bryan L. DREADDs for Neuroscientists, Neuron (2016) 89, 683-694.
[6] Armbruster, B. N.; Li, X.; Pausch, M. H.; Herlitze, S.; Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand, Proceedings of the National Academy of Sciences (2007) 104, 5163-5168.
[7] Silva, B. A.; Mattucci, C.; Krzywkowski, P.; Murana, E.; Illarionova, A.; Grinevich, V.; Canteras, N. S.; Ragozzino, D.; Gross, C. T. Independent hypothalamic circuits for social and predator fear, Nat Neurosci (2013) 16, 1731-1733.
[8] Errico, F.; D'Argenio, V.; Sforazzini, F.; Iasevoli, F.; Squillace, M.; Guerri, G.; Napolitano, F.; Angrisano, T.; Di Maio, A.; Keller, S.; Vitucci, D.; Galbusera, A.; Chiariotti, L.; Bertolino, A.; de Bartolomeis, A.; Salvatore, F.; Gozzi, A.; Usiello, A. A role for D-aspartate oxidase in schizophrenia and in schizophrenia-related symptoms induced by phencyclidine in mice, Transl Psychiatry (2015) 5, e512.
[9] Ferrari, L.; Turrini, G.; Crestan, V.; Bertani, S.; Cristofori, P.; Bifone, A.; Gozzi, A. A robust experimental protocol for pharmacological fMRI in rats and mice, Journal of Neuroscience Methods (2012) 204, 9-18.
Figures