0106
An exploration of quantitative physiological multi-modal in-vivo imaging of the human placenta1Biomedical Engineering Department, King's College London, London, United Kingdom, 2Centre for the Developing Brain, London, United Kingdom, 3Centre for Medical Image Computing, University College London, London, United Kingdom, 4Centre for the Developing Brain, King's College London, London, United Kingdom
Synopsis
The crucial role of the placenta in successful pregnancies is the transfer of oxygen within functional units – cotyledons. However, current screening falls short of visualizing this in-vivo. This study explores a multi-model in-vivo MRI acquisition able to visualize and depict a range of spatial and temporal processes and the underlying micro-structure. Diffusion characteristics such as Mean Diffusivity and fractional anisotropy, quantitative T2* maps, temporal characteristics and the depiction of vasculature allow insights and can be applied to a range of research questions.
Background
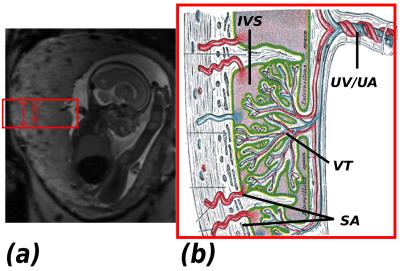
The transfer of oxygen to the growing fetus is a crucial function of the human placenta. It occurs within the key functional lobules (Fig.1). Developmental abnormalities that cause variations in the properties of the maternal blood supply or decreased fetal villous surface area, have been hypothesized from ex-vivo studies and in models (1) to result in major pregnancy complications such as fetal growth restriction and early onset pre-eclampsia. However, current available screening (e.g. Ultrasound) fails to access internal placental function, while previous MRI studies (2,3) focused on individual modalities. This study presents a first multi-model MR imaging capability combining anatomical and micro-structural information seeking to inform on both spatial and temporal oxygenation variation within the placenta.Methods
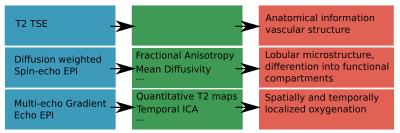
A bespoke quiet EPI sequence was used to acquire both multi-echo GE (meGE) and diffusion-weighted Spin Echo (dSE) data on the placenta and adjacent myometrium in 4 healthy pregnant volunteers on a clinical 3T Philips Archieva scanner using the 32-channel cardiac coil. Scanning parameters were: resolution 2.2mm3, FOV 310x310x[55-77] mm33. The parameters for meGRE were TE = 20,62,104,146ms, TR = 8.1s, 50 dynamics, TA=6:48., for dSE five b=0 images, three images for each of b=15, 25, 80, 115, 206, 246, 346 s/mm2, and eight images each for b=40, 400, 1000, 2000 s/mm2, TA= 3:55. One T2 TSE scan was acquired for anatomical reference and to visualise maternal arteries (TA= 1:21). Time series of quantitative T2* maps were calculated from the meGE data after motion-correction using MCFLIRT (5), and analysed with MELODIC Independent component analysis (ICA) (4). The dSE data was processed using Camino (6) to obtain Mean Diffusivity (MD) and fractional anisotropy (FA) information to assess the microstructure. The process is illustrated in Fig. 2.Results
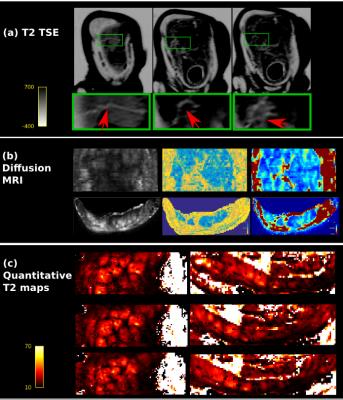
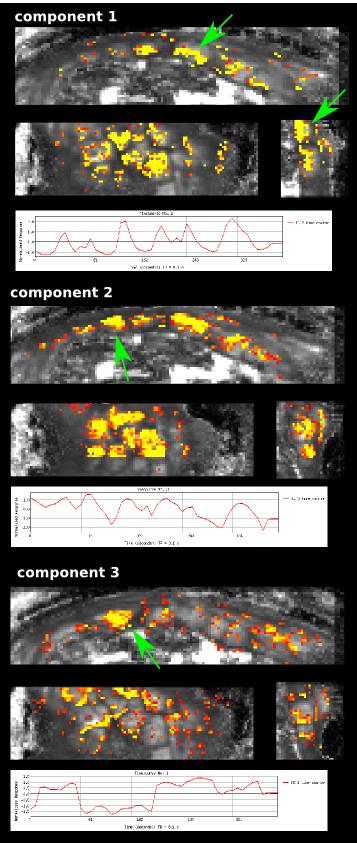
Data from all modalities is depicted in Fig.3 for one subject, showing the maternal uterine and spiral arteries feeding the lobule centres in bright white (inverted color scale) in Fig.3a. The arrows indicate from left to right the maternal circulation from uterine to radial to spiral arteries. The dMRI data in Fig.3b differentiates the maternal basal plate and the lobules as functional compartments through the spatial variation in diffusivity and anisotropy and shows differentiation within the lobule. Additionally, the relaxation time maps in Fig.3c depict T2* gradations co-localised with functional units, consistent with decreasing concentration in oxy-haemoglobin from the lobule centres to their periphery, and perhaps signifying oxygen exchange from the maternal to the fetal compartments. Finally, the ICA components, shown in Fig.4 together with the corresponding time courses, may demonstrate spatial components of placental function. The first component hints at rhythmic inflow patterns on the fetal placental side, component 2 with a slightly different time series potentially depicts spatially inhomogeneous bursts of maternal blood (7). The 3rd component shows a different time series and a single clearly pronounced area of activation. Corresponding results were seen for all modalities across all subjects.Discussion and Outlook
The proposed multi-modal functional placental MRI acquisition approach shows encouraging capability to reveal potential signatures of placental function at many levels within the functional lobules. It could provide a foundation to explore biologically hypothesised physiology in-vivo, but will need further testing, particularly in pathological pregnancies with abnormal placental function as evidenced by Doppler blood flow studies and fetal and maternal clinical state.Acknowledgements
MRC strategic funds (MR/K006355/1), GSTT BRC and NIH-funded Placenta imaging Project: project number 1U01HD087202-01.References
(1) Burton GJ1, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009 Jun;30(6):473-82. doi: 10.1016/j.placenta.2009.02.009. Epub 2009 Apr 17.
(2) Moore, R. J., Strachan, B. K., Tyler, D. J., Duncan, K. R., Baker, P. N., Worthington, B. S., Johnson, I. R. & Gowland, P. A. In utero perfusing fraction maps in normal and growth restricted pregnancy measured using IVIM echo-planar MRI. Placenta 21, 726–732 (2000).
(3) Sinding M1, Peters DA2, Frøkjær JB3, Christiansen OB4, Uldbjerg N5, Sørensen A6. Reduced placental oxygenation during subclinical uterine contractions as assessed by BOLD MRI. Placenta. 2016 Mar;39:16-20. doi: 10.1016/j.placenta.2015.12.018. Epub 2016 Jan 5.
(4) Beckmann, C. F., and Smith, S. M. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 23, 137–152. doi:10.1109/TMI.2003.822821
(5) Jenkinson, M., Bannister, P., Brady, M., and Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. doi:10.1006/nimg.2002.1132
(6) Cook, P. A., Bai, Y., Seunarine, K. K., Hall, M. G., Parker, G. J. & Alexander, D. C. Camino: Open-Source Diffusion-MRI Reconstruction and Processing. 14th Sci. Meet. Int. Soc. Magn. Reson. Med. 14, 2759 (2006). 7 Frias AE1, Schabel MC, Roberts VH, Tudorica A, Grigsby PL, Oh KY, Kroenke CD. Using dynamic contrast-enhanced MRI to quantitatively characterize maternal vascular organization in the primate placenta. Magn Reson Med. 2015 Apr;73(4):1570-8. doi: 10.1002/mrm.25264. Epub 2014 Apr 18.
Figures