0105
Parametric Mapping of Oxygen Activity in Human Placenta across Gestation using in utero BOLD imaging1Children's Hospital Los Angeles, Los Angeles, CA, United States, 2Rudi Schulte Research Institute
Synopsis
We present here, for the first time, parametric maps of oxygen activity in normal human placenta using in utero functional MR imaging. Our method highlights anatomical and gestational age dependent patterns in placental activity. These maps can be used to gain insight into normative placental function and identifying insufficient or abnormal placental functioning at various points in gestation.
Introduction
Our current understanding of placental development and function is based on animal imaging [4] and ex-vivo studies of placenta obtained after delivery or interrupted pregnancies. Previous human imaging studies were restricted to understanding hyperoxygentation[1,2] or adverse developmental conditions such as fetal growth restriction (FGR), placental previa, placenta accrete, etc. [5]. These studies strongly indicate that improved in-vivo delineation of vasculogenesis and angiogenesis of the placenta has the potential to provide better insight into the pathogenesis of placental dysfunction. By leveraging non-invasive, high-resolution imaging capabilities of in utero fetal MRI, we present a spatiotemporal analysis of normative fetoplacental oxygenation patterns at various time points in gestation. We hypothesize that (a) spatial variance of BOLD placental signal would age-dependent , and (b) that serial parametric maps of BOLD signal would reveal important anatomic insights about the feto-placental and maternal circulation.Methods
We conducted a prospective two-site study of placental development in which 20 maternal subjects with normal pregnancies were recruited between 26- 37 gestational weeks (GW). Images were acquired using 3T Philips Ingenia or Siemens Skyra machines. Over a 5- 10 minute total acquisition time, BOLD images (1.5 mm x 1.5 mm x 4 mm) were acquired using an EPI sequence in blocks of 60 images with TR/TE = 3000/35 ms, flip angle = 90°. In addition, a high resolution T2 weighted images (1 mm x 1 mm x 3 mm) were acquired using a 3D FFE sequence (TR/TE = 3.1/1.6 ms, flip angle = 75°). BOLD images were processed using the "Functional MRI of the Brain" Software Library (FSL) [6]. The BOLD signal was motion corrected, coregistered to the T2 images and filtered to remove physiological noise such as cardiac, breathing and metabolic fluctuations. An F test was used to test the relationship between spatial signal variations and gestational age. To compare oxygenation levels within the placenta, we computed the normalized BOLD values after averaging the amplitude of the BOLD signal over the duration of the study for each time point.Results
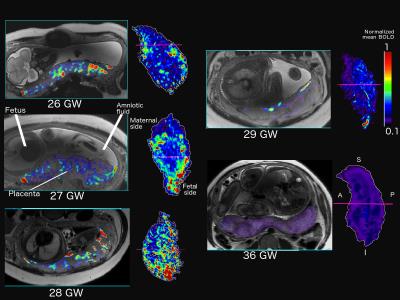
We found that the spatial variance of the BOLD signal was age dependent (F= 2.25, p<0.001). We then generated parametric maps of oxygen activity in a subset of fetuses at various gestational ages. Figure 1 shows the mean BOLD signal values across the placenta between 26 and 36 GW. The parametric mapping delineates two distinct regions of high oxygenation activity, corresponding to the fetal side (adjacent to the site of umbilical cord attachment) and the maternal side (along the uterine wall). The regions of high activity also occurred in specific clusters. The size and number if these high-activity regions increased from 26 to 28 GW. We then observed a drop in the number and size of these regions at 29 GW with a further decrease at 36 GW.Discussion
Our results clearly indicate that oxygenation is spatially heterogenous across the placenta with oxygen activity concentrated at specific anatomical locations. The spatial variance in oxygen activity is also age-dependent. The reduction in size and number of high activity clusters at 29 GW and beyond correlates with the involution of the placenta in the third trimester leading to birth [3]. Lower BOLD signal values in the middle of the placenta could correspond to the lack of deoxyhemoglobin as these regions only transport oxygen to the umbilical cord [7]. The oxygenation maps provide a baseline for how oxygen activity occurs and changes over gestation giving us a better understanding of fetoplacental haemodynamics and placental transfer. They may also be used to identify abnormal oxygenation patterns in a placenta thereby acting as a marker for early detection of FGR or insufficient placental function.Conclusion
There are age-dependent, spatial variances of BOLD signal in the placenta which may correlate with angiogenesis. Using parametric mapping of placental BOLD signal, we have demonstrated that placental oxygenation activity is concentrated at specific anatomical locations associated with feto-maternal oxygen exchange. The non-invasive and repeatable methods presented here may facilitate better predictions of placental dysfunction in high-risk pregnancies and inform perinatal care.
Acknowledgements
No acknowledgement found.References
1. Sørensen, A., et al. "Changes in human placental oxygenation during maternal hyperoxia estimated by blood oxygen level-dependent magnetic resonance imaging (BOLD MRI)." Ultrasound in Obstetrics & Gynecology42.3 (2013): 310-314.
2. Andescavage, Nickie Niforatos, Adre du Plessis, and Catherine Limperopoulos. "Advanced MR imaging of the placenta: Exploring the in utero placenta–brain connection." Seminars in perinatology. Vol. 39. No. 2. WB Saunders, 2015.
3. Kara, Simay A., et al. "Placental aging, fetal prognosis and fetomaternal Doppler indices." European Journal of Obstetrics & Gynecology and Reproductive Biology 82.1 (1999): 47-52.
4. Aimot-Macron, S., et al. "In vivo MRI assessment of placental and foetal oxygenation changes in a rat model of growth restriction using blood oxygen level-dependent (BOLD) magnetic resonance imaging." European radiology23.5 (2013): 1335-1342.
5. Chalouhi, Gihad E., et al. "Fetoplacental Oxygenation in an Intrauterine Growth Restriction Rat Model by Using Blood Oxygen Level–Dependent MR Imaging at 4.7 T." Radiology 269.1 (2013): 122-129.
6. S.M. Smith, M. Jenkinson, M.W. Woolrich, C.F. Beckmann, T.E.J. Behrens, H. Johansen-Berg, P.R. Bannister, M. De Luca, I. Drobnjak, D.E. Flitney, R. Niazy, J. Saunders, J. Vickers, Y. Zhang, N. De Stefano, J.M. Brady, and P.M. Matthews. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(S1):208-19, 2004
7. Poston, Lucilla. "The control of blood flow to the placenta." Experimental physiology 82.2 (1997): 377-387.
Figures