0101
Ex vivo MRI evaluation of vulvar cancer to predict resection margins in fresh wide local excision specimens: a pilot study1Radiology and Nuclear Medicine, Radboud university medical center, Nijmegen, Netherlands, 2Obstetrics and Gynaecology, Radboud university medical center, Nijmegen, Netherlands, 3Pathology, Radboud university medical center, Nijmegen, Netherlands
Synopsis
Currently there’s no accurate and topical peroperative information available on the margin status of wide local resection specimens containing vulvar cancer. In this pilot study we performed a qualitative image evaluation of ex vivo 7T MR images acquired of fresh specimens of the vulva containing vulvar cancer using different MRI sequences. High resolution T2 weighted images obtained the highest score for image quality, visibility of the tumor, and visibility of the transition between the epidermis and the resection surface.
Introduction
Vulvar cancer is a relatively uncommon
disease accounting for approximately 5% of all gynaecological malignancies1,2.
Currently, wide local excision (WLE) is the preferred treatment of the primary tumor
in early stage disease3–7.
The success of this approach depends on the achievement of tumor free margins
which are considered the most important predictive factor for local recurrences8–11.
The challenge in treatment of vulvar cancer is to keep surgical margins large
enough to minimize the chance of local recurrence, but at the same time,
preserve as much genital tissue as possible to diminish morbidity to urethra,
clitoral tissue or anal sphincter. The surgeon is hampered by the fact that no
accurate and topical information on the margin status is available during or
direct after the surgery. The goal of this pilot study was to investigate
whether ex vivo 7T MRI is a feasible technique to visualize vulvar cancer and the
transition between epidermis and resection surface in fresh WLE specimens.Methods
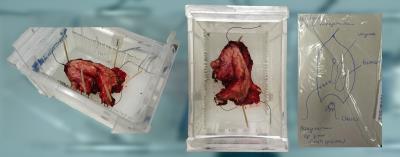
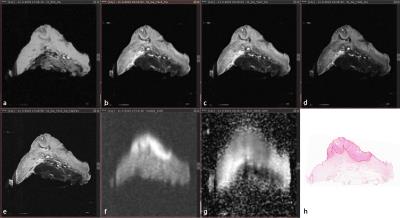
Approval of our Institutional Review Board and written informed consent from all patients (n=11) were obtained. Consecutive patients who had biopsy proven vulvar cancer and who were scheduled for WLE were prospectively included. Directly after surgery the entire, intact, vulva specimen was positioned in a specifically designed container and was scanned using a 7 Tesla preclinical MR scanner interfaced to a Siemens console. Axial T1 weighted (T1W, FLASH, TE/TR = 3/400, voxel size = 0.31x0.31x1 mm), T2 weighted (T2W, TSE, TE/TR = 13, 26, 53/6,090-12,470, voxel size = 0.31x0.31x1 mm), and diffusion weighted (DW, HASTE, TE/TR = 53/2,130, voxel size = 0.63x0.63x1 mm, b-values = 0, 100, 500, 1000, 1200 s/mm2) images were acquired. Furthermore, one T2W image set (TSE, TE/TR = 14/6,400-13,120, voxel size = 0.16x0.16x1 mm) was acquired in extra high resolution. Apparent diffusion coefficient (ADC) maps were calculated. A radiologist performed qualitative evaluation of the general image quality of each of the vulva specimens. The datasets were assessed using the following 5-point rating scale12: 1, very poor or non-diagnostic quality images; 2, low image quality that degraded confidence in diagnosis; 3, moderate image quality sufficient for diagnosis; 4, good image quality; 5, excellent image quality enabling visualisation of even small structures. Next to general image quality, the visibility of the tumor and the transition between epidermis and resection surface were qualitatively assessed by applying the following scale: 1, very poor; 2, low; 3, sufficient; 4, good; 5, excellent. The median, interquartile range and extreme values of the results were calculated and represented in box plots.Results
Ex vivo MRI of WLE specimen produced
good quality images at which vulvar cancer was well visible. The transition
between epidermis and resection surface was sufficiently to very poorly visible.
In general, the T2W sequences, particularly the T2W-14 HighRes sequence,
demonstrated the highest median scores for image quality (median=5), visibility
of the tumor (median=5), and visibility of the epidermis-resection edge
transition (median=4). The scores were not dependent on the used echo time,
except for the visibility of the epidermis-resection edge transition that was
assigned a lower median score at the T2W-53. The b1000 DW sequence and the
ADC-map demonstrated good results for tumor visualisation (medians= 4; 4), yet
visualisation of the epidermis-resection surface transition was very bad (medians=1;
1). Although the T1W sequence provided good image quality (median=4), it did
not perform well in visualization of the tumor and epidermis-resection edge
transition (medians=3; 1.5).Discussion
It is feasible to perform ex vivo MR
images from WLE specimens containing vulvar cancer. Similar to low field
strength MRI, vulvar cancer has low signal intensity on T1W images and
moderate-to-high signal intensify on T2W images13.
Furthermore, vulvar cancer demonstrates diffusion restriction probably as a
result a higher cellular density. Limitations are the
small number of patients and the fact that this was a one reader study. This
precursory study investigated whether ex vivo MRI of WLE specimens was feasible
at all and what MR sequence results in the best image quality, tumor visibility
and visibility of the transition between epidermis and resection surface. The
results of this study will be employed in a succeeding study that will
investigate whether ex vivo MRI can be applied to localize vulva cancer and
subsequently predict resection margins in fresh WLE specimens.Conclusion
Ex vivo MRI of fresh WLE specimens containing vulvar cancer is a feasible. High resolution T2W images provided the highest image quality and have proved to be the best sequence to visualize vulvar cancer and the transition between epidermis and resection surface.Acknowledgements
Ex vivo MRI was performed using equipment of the Preclinical Imaging Centre (PRIME) within the Radboud university medical center, Nijmegen, the Netherlands.References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics , 2015 . CA Cancer J Clin. 2015;65(1):5–29.
2. Lai J, Elleray R, Nordin A, et al. Vulval cancer incidence, mortality and survival in England: age-related trends. BJOG An Int J Obstet Gynaecol. 2014;121(6):728–738.
3. Ansink A, van der Velden J. Surgical interventions for early squamous cell carcinoma of the vulva. Cochrane Database Syst Rev. 2000;(2):CD002036.
4. de Hullu JA, van der Zee AGJ. Surgery and radiotherapy in vulvar cancer. Crit Rev Oncol Hematol. 2006;60(1):38–58.
5. Stehman FB, Look KY. Carcinoma of the vulva. Obstet Gynecol. 2006;107(3):719–733.
6. Micheletti L, Preti M. Surgery of the vulva in vulvar cancer. Best Pract Res Clin Obstet Gynaecol. 2014;28(7):1074–1087.
7. Alkatout I, Schubert M, Garbrecht N, et al. Vulvar cancer: epidemiology, clinical presentation, and management options. Int J Womens Health. 2015;7:305–313.
8. Heaps JM, Fu YS, Montz FJ, Hacker NF, Berek JS. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol. 1990;38(3):309–314.
9. de Hullu JA, Hollema H, Lolkema S, et al. Vulvar carcinoma: The price of less radical surgery. Cancer. 2002;95(11):2331–2338.
10. Chan JK, Sugiyama V, Pham H, et al. Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: A multivariate analysis. Gynecol Oncol. 2007;104:636–641.
11. Rouzier R, Haddad B, Plantier F, Dubois P, Pelisse M, Paniel BJ. Local relapse in patients treated for squamous cell vulvar carcinoma: Incidence and prognostic value. Obstet Gynecol. 2002;100(6):1159–1167.
12. Schueller-Weidekamm C, Schaefer-Prokop CM, Weber M, Herold CJ, Prokop M. CT angiography of pulmonary arteries to detect pulmonary embolism: improvement of vascular enhancement with low kilovoltage settings. Radiology. 2006;241(3):899–907.
13. Sohaib S, Richards P, Ind T, et al. MR imaging of carcinoma of the vulva. 2002;(February):373–377.
Figures