0097
Amide proton transfer magnetic resonance imaging of uterine endometrial cancer: Association with histologic grade1Department of Radiology Informatics and Network, Kyushu University, Fukuoka, Japan, 2Department of Clinical Radiology, Kyushu University, 3Department of Gynecology & Obstetrics, Kyushu University, 4Philips Research
Synopsis
The histologic grade of endometrioid adenocarcinoma (EMCA) is one of the important factors in choosing a treatment plan. Currently, needle biopsy or surgical resection is necessary to diagnose the histological grade; a less invasive procedure is strongly desired. In this study, we evaluated the utility of amide proton transfer (APT) imaging in estimating the histologic grade of EMCA. APT signal intensities (SIs) of EMCA increased in accordance with the progression of histologic grade. APT SIs of high-grade EMCA were significantly higher than those of low-grade EMCA. APT imaging has potential as a biomarker for histologic grade of EMCA.
Introduction
Amide proton transfer (APT) imaging is a novel magnetic resonance imaging (MRI) technique. APT imaging is based on the chemical exchange between bulk water protons and labile solute protons, such as those bearing NH- and OH- groups. Previous studies have reported the clinical utility of APT imaging to estimate the aggressiveness of brain, lung and prostate tumors. However, there has been no report discussing the potential of APT imaging for the evaluation of uterine tumor aggressiveness. We hypothesized that APT imaging might be useful for non-invasive histological evaluation of endometrioid adenocarcinoma (EMCA, the most common type of uterine endometrial cancer), to replace current biopsy methods. The purpose of this study was to investigate the clinical feasibility of using APT imaging to evaluate the histologic grade of EMCAMaterials and Methods
A total of 35 patients (age = 59.2 ± 12.1 years) suspected of having EMCA were enrolled in this study. MR examinations were performed on a 3.0-Tesla MRI system (Achieva 3.0T TX, Philips Healthcare, Best, the Netherlands). APT imaging was performed in addition to conventional MRI such as T1WI, T2WI, DWI and DCE-MRI. Twenty-nine of the 35 patients underwent surgery and received pathological confirmation of EMCA and their histologic grades. Of the 29 carcinomas, 9 were well-differentiated (Grade 1), 11 were moderately differentiated (Grade 2), and 9 were poorly differentiated (Grade 3). The other 6 patients did not undergo surgery because no malignant cells were discovered on needle biopsies, and follow-up examinations showed no progressive disease. We defined these 6 patients without EMCA as the no-malignancy group. The MR parameters of APT imaging were as follows: 2D-TSE sequence with driven equilibrium refocusing, TR/TR = 5000/6 ms, FOV = 230×230 mm2, resolution = 1.8×1.8×5 mm3, 25 saturation frequency offsets = -6 to 6 ppm (step of 0.5 ppm) and ω0 = -1560 ppm, saturation pulse length = 0.5, 1.0 and 2.0 s, B1rms = 2.0 μT. δB0 maps were acquired separately for a δB0 correction. The APT signal intensity (APT SI) was defined as: MTRasym = {S[-3.5 ppm]-S[+3.5 ppm]}/S0}×100 (%). APT SIs with saturation pulse length of 0.5, 1.0 and 2.0 s were defined as APT0.5, APT1.0 and APT2.0, respectively. Apparent diffusion coefficient (ADC) maps were generated referring to the SIs of DWI with b-values of 0 and 1000 s/mm2. APT SIs and ADC values of the endometrium or endometrial lesions were calculated after drawing regions of interest on the APT imaging or ADC maps. The average APT SIs, and minimum and mean ADC values among the four groups were compared using one-way analysis of variance with Tukey’s HSD post hoc test. In addition, Spearman's rank correlation coefficients (Spearman’s ρ) were calculated to evaluate the relationship between the APT SIs and the histologic grade, and between ADC values and the histologic grade.Results
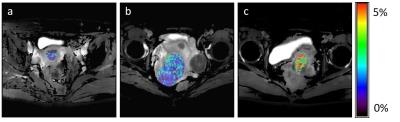
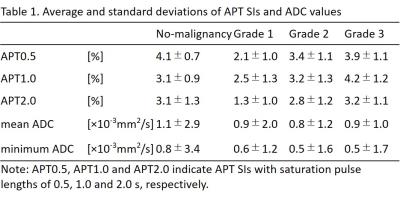
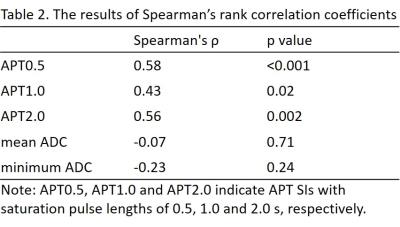
The average ± standard deviations of APT SIs and minimum and mean ADC values are shown in Table 1. APT0.5 and APT2.0 showed significant differences between the no-malignancy group and Grade 1, between Grades 1 and 2, and between Grades 1 and 3 (p<0.05). APT1.0 showed a significant difference between Grades 1 and 3 (p<0.05). The mean ADC showed a significant difference between the no-malignancy group and Grade 2 (p<0.05). Minimum ADC values showed significant differences between the no-malignancy group and Grade 2 and between the no-malignancy group and Grade 3 (p<0.05). Other pairwise comparisons were not statistically significant. The results of Spearman’s rank correlation coefficients are shown in Table 2. All APT SIs showed significant positive correlations with histologic grade (p<0.05). Mean and minimum ADC values showed negative correlations with the histologic grade although these correlations were not statistically significant. Representative APT images of each histologic grade in EMCA are shown in Figure 1.Discussion and Conclusion
Our results showed that APT SIs of EMCA were significantly correlated to the histologic grade, but ADC values of EMCA were not. Overall, high-grade EMCA showed significantly higher APT SIs compared to low-grade EMCA. These results were consistent with the significant relationship between tumor aggressiveness and APT SIs reported in brain, lung and prostate tumors. High APT SIs of high-grade EMCA might reflect increased protein synthesis and abundant content of extracellular and/or intracellular proteins compared to low-grade EMCA. We have no plausible explanation for the inconsistency of our results for ADC values in EMCA with previous results. We conclude that APT imaging has potential as a biomarker to estimate the aggressiveness of EMCA.Acknowledgements
No acknowledgement found.References
1. Zhou J, van Zijl PC. Chemical exchange saturation transfer imaging and spectroscopy. Progr. NMR Spectr. 2006;48:109–136.
2. Togao O, Yoshiura T, Keupp J, et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro-oncol 2014;16:441-448.
3. Ohno Y, Yui M, Koyama H, et al. Chemical Exchange Saturation Transfer MR Imaging: Preliminary Results for Differentiation of Malignant and Benign Thoracic Lesions. Radiology 2016;279:578–589.
4. Takayama Y, Nishie A, Sugimoto M, et al. Amide proton transfer (APT) magnetic resonance imaging of prostate cancer: comparison with Gleason scores. MAGMA 2016;29:671–679.
5. Seo JM, Kim CK, Choi D, et al. Endometrial Cancer: Utility of Diffusion-Weighted Magnetic Resonance Imaging With Background Body Signal Suppression at 3T. J. Magn. Reson. Imaging 2013; 37:1151–1159.
6. Inoue C, Fujii S, Kaneda S, et al. Correlation of Apparent Diffusion Coefficient Value With Prognostic Parameters of Endometrioid Carcinoma. J. Magn. Reson. Imaging 2015;41:213–219.
Figures