0096
A Flexible Technique for Flow-Sensitive Fat-Suppressed High-Resolution Peripheral Nerve Imaging1Global MR Applications & Workflow, GE Healthcare, Menlo Park, CA, United States, 2Global MR Applications & Workflow, GE Healthcare Japan, Hino, Japan, 3Global MR Applications & Workflow, GE Healthcare, Madison, WI, United States, 4Global MR Applications & Workflow, GE Healthcare, Waukesha, WI, United States, 5Department of Radiology, Stanford University, Stanford, CA, United States
Synopsis
We developed a flow-sensitive 3D fast spin echo pulse sequence with Dixon-based water-fat separation and compressed sensing for robust and efficient peripheral nerve imaging. Outer volume suppression allows shorter scan times by limiting spatial encoding of the FOV to the anatomy of interest without aliasing concerns. In addition, it improves the performance of spectrally-selective fat suppression methods, that can be advantageous for very high resolution imaging but are typically hampered by B0 inhomogeneity, by allowing shimming over smaller regions. Preliminary data showed good delineation of peripheral nerves in different anatomies, with adequate resolution and clinically feasible acquisiton times.
Introduction
MR neurography is increasingly being used for the clinical assessment of peripheral nerves1. High-resolution, flow-sensitive, T2-weighted, volumetric imaging with fat suppression has been shown to be a promising technique for peripheral nerve imaging2. Short Time Inversion Recovery (STIR) is often preferred over chemically selective fat suppression for being intrinsically more robust to off-resonance, despite the lower signal-to-noise ratio (SNR). Flow suppression can further degrade SNR, especially when a very low VENC (Velocity ENCoding) is selected. The additional requirement for high isotropic resolution usually entails long acquisition times, especially for brachial and lumbar-sacral plexus, where large anatomical coverage, both in-plane and through-plane, is required. Here we propose a flexible 3D-FSE-based acquisition with Motion-Sensitive Driven Equilibrium3 (MSDE) and either Dixon-based water-fat separation or Adiabatic SPectral Inversion Recovery (ASPIR) to achieve robust fat suppression while maintaining SNR efficiency. In addition, Outer Volume Suppression (OVS) and Compressed Sensing (CS), together with parallel imaging, are used to accelerate the acquisition by limiting the encoded FOV to the anatomy of interest (OVS) and by allowing higher data undersampling than with parallel imaging alone (CS).Methods
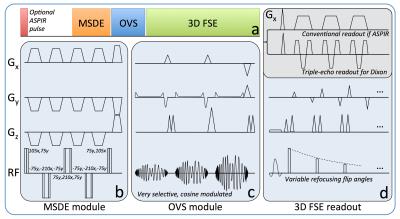
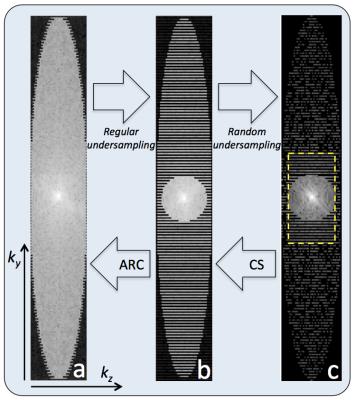
A schematic diagram of the proposed prototype pulse sequence is shown in Figure 1. The OVS module was played out immediately before the start of the 3D FSE readout to minimize outer-volume T1 recovery and consisted of three high-bandwidth (8kHz), quadratic-phase, cosine-modulated RF pulses followed by spoiler gradients for simultaneous outer volume suppression4 (Fig. 1a, c). Flip angles were optimized to compensate for T1 and B1 imperfections. The MSDE module consisted of a (105°x, 75°y) excitation pulse followed by three (-75°y, -210°x, -75°y) refocusing pulses and a (75°y, 105°x) tip-up pulse to restore the magnetization along the longitudinal axis (Fig. 1b). Motion sensitizing gradients (VENC=1.5-2.0cm/s) were simultaneously applied on all 3 axes and either side of each refocusing pulse. When ASPIR was used for fat suppression, the MSDE and OVS modules were played during the ASPIR inversion recovery time (~170ms at 3T) and no modification was made to the FSE readout. For Dixon-based fat suppression, a single-TR, triple-echo technique with joint water-fat estimation was used5 (Fig. 1d). Variable refocusing flip angles were used for the 3D FSE readout to reduce SAR (Specific Absorption Rate) and T2-induced blurring6. CS and data-driven parallel imaging (ARC) were applied sequentially, as previously demonstrated7 (Figure 2). All imaging was performed at 3T (MR750, GE Healthcare, Waukesha, WI) using dedicated receive-only array coils for signal reception (16 channels for brachial plexus, 20 for lumbar sacral plexus, 8 for ankle). Different anatomical regions in healthy subjects and patients were imaged using protocols tailored to the specific anatomy of interest to maximize nerve conspicuity and overall image quality, while minimizing scan time. IRB approval and informed consent were obtained.Results and Discussion
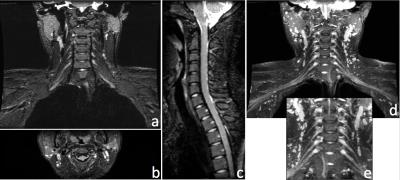
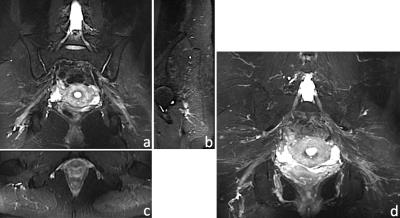
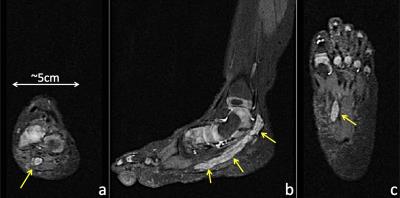
Figure 3 shows 1.2×1.0×1.2mm3 brachial plexus images acquired in a healthy volunteer, using MSDE, Dixon-based fat suppression, OVS and CS. In this case, the use of OVS and CS reduced scan time by 36%, from 4min 15s to 2min 43s, while maintaining good SNR. Uniform fat suppression and good flow suppression were obtained, as evidenced by the orthogonal reformats (Fig. 3a-c) and MIP (Maximum Intensity Projection) reconstruction (Fig. 3d-e). The results of a similar acquisition (1.2mm3 isotropic), using ASPIR instead of Dixon, is shown in Figure 4. In this case, OVS alone gave a 36% scan time reduction with respect to conventional phase oversampling. CS (1.4x udersampling) provided an extra 29% scan time reduction over conventional 2x ARC undersampling, resulting in a ~5min scan. While the quality of fat suppression and nerve conspicuity were similar to the brachial plexus case, several small vessels were still visible in the lumbosacral area, especially in the MIP reconstruction, probably due to VENC being too high for this particular anatomy. Figure 5 shows axial (5a), sagittal (5b) and coronal (5c) reformats of the foot of a 19 month old baby, where fat suppression is often challenging. This dataset (0.5×0.6×0.8mm3) was acquired using a dual-TR Dixon approach (readout shifted every other TR). Neither OVS or CS were used, resulting in an 8min scan. Uniform fat suppression and excellent delineation of pathology were obtained.Conclusion
We developed a flow-sensitive, volumetric, FSE-based pulse sequence for peripheral nerve imaging. Depending on the anatomical region, either a Dixon-based water-fat separation method or ASPIR are used for fat suppression. OVS and CS allow to speed up the acquisition significantly. Good nerve delineation and overall image quality were demonstrated in healthy subjects and a 19 month old pediatric patient.Acknowledgements
No acknowledgement found.References
1. Chhabra A, Andreisek G, Soldatos et al. MR Neurography: Past, Present, and Future. American Jornal of Roentgenology. 2011; 197(3): 583-591.
2. Kasper JM, Wadhwa V, Scott KM et al. SHINKEI--a novel 3D isotropic MR neurography technique: technical advantages over 3DIRTSE-based imaging. Eur Radiol. 2015; 25(6): 1672-1677.
3. Koktzoglou I, Li D. Diffusion-prepared segmented steady-state free precession: application to 3D black-blood cardiovascular magnetic resonance of the thoracic aorta and carotid artery walls. J Cardiovasc Magn Reson. 2007; 9: 33–42.
4. Banerjee S, Han M, Chen W et al. Reduced Field-Of-View Imaging with 3D Variable Flip Angle Fast Spin Echo-Feasibility in MRI of Orbits. Proceedings 23rd Annual Meeting ISMRM. 2015; p.2309.
5. Ma J, Son JB, Zhou Y et al. Fast Spin-Echo Triple-Echo Dixon (fTED) Technique for Efficient T2-Weighted Water and Fat Imaging. Magnetic Resonance in Medicine. 2007; 58: 103–109.
6. Busse RF, Hariharan H, Vu A et al. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med. 2006; 55(5): 1030-1037.
7. Beatty PJ, King KF, Marinelli L et al. Sequential Application of Parallel Imaging and Compressed Sensing. Proceedings 17th Annual Meeting ISMRM. 2009; p.2824.
Figures