0094
Magnetic Resonance Imaging of the Functional Anatomy of the Oblique Muscles in Patients with Primary Oblique Overaction1Department of Optometry and Visual Science, West China Hospital, Sichuan University, Chengdu, People's Republic of China, 2Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Department of Ophthalmology, West China Hospital, Sichuan University, Chengdu, People's Republic of China, 4NeuroRepair Department, Mossakowski Medical Research Centre PAS, Warsaw, Poland
Synopsis
The cause of primary eye movement abnormality is unknown. The functional MRI of superior and inferior oblique muscles was instrumental to investigate the cause of overaction. We have shown similar size of superior oblique muscle in patients and controls in the resting state, while the MRI performed during gazes revealed differences in the contractility, what suggests the abnormal innervation as a cause of primary superior oblique muscles. In contrast, the inferior oblique muscle was larger in resting state, without a difference in contractility what indicates the hypertrophy as a basis for primary inferior oblique muscle overaction.
Purpose
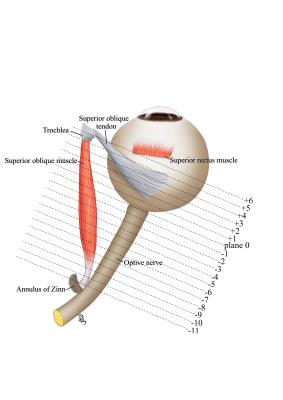
Oblique muscle overaction is typically diagnosed based on physical examination, and interferes with binocular vision and quality of life. However, the clinical examination poorly distinguishes SO overaction from other scenarios, such as heterotopic rectus extraocular muscles (EOM) pulleys, or other orbital or structural EOM abnormalities (1). The current progress in magnetic resonance imaging (MRI) permits addressing the functional anatomy of the eye, which was exploited in the studies of SO palsy (2-3). We aim to quantitatively determine the size and contractility of the superior oblique muscle and inferior oblique muscle in primary superior oblique overaction (PSOOA) and primary inferior oblique muscle overaction (PIOOA), to facilitate the differential diagnosis, thus open the way for more targeted treatment.Methods
A prospective, observational study was conducted on twelve patients with PSOOA, 7 cases with PIOOA, and 10 healthy,Results and Discussion
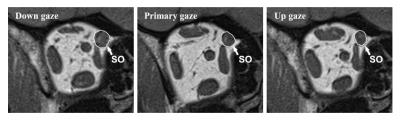
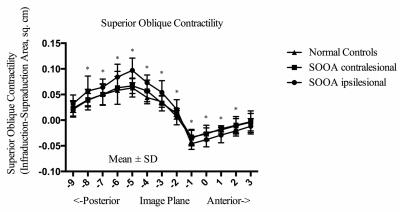
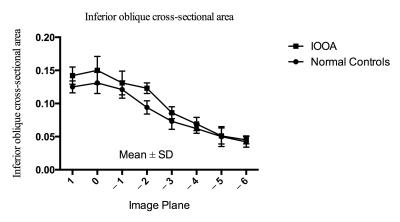
In the primary position, the greatest SO cross-sections occurred about midorbit, 6 mm posterior to the globe-optic nerve junction. In down gaze, the SO cross-sectional area increased, and the plane in which the maximum cross-sectional area observed was more posterior. In up gaze, the SO cross-sectional area decreased, and the plane in which the maximum cross-sectional area observed was more anterior (Fig. 3). There was no difference between the ipsilesional (affected eye), contralesional (unaffected eye) and normal SO muscle cross-sections: 0.176 ± 0.018 cm2, 0.175 ± 0.005 cm2, and 0.173 ± 0.015 cm2, respectively (P=0.82). The maximum contractility of SO muscle on the ipsilesional (affected) side was 0.097 ± 0.024 cm2, and was different than on the contralesional (unaffected) side: 0.067 ± 0.015 cm2 and in control subjects: 0.063 ± 0.018 cm2 (P=0.0002) (Figure 4). For PIOOA, compared IO of the patient group and control group of subjects, ipsilesional IO maximum cross-sectional area (0.150 ± 0.021 cm2) was greater than that of normal IO maximum cross-sectional area (0.131 ± 0.016 cm2) (P=0.023) (Fig. 4). However, ipsilesional IO maximum contractility (0.052 ± 0.013 cm2) did not differ from that of normal subjects (0.0430 ± 0.011 cm2) (P=0.080). It is reported that the cause of EOM overaction includes excessive innervation, an increase in muscle’s cross-sectional area (hypertrophy), and changes in muscle fiber types within the muscle (1, 4). It seems that muscle hypertrophy is the likely reason for PIOOA, not for the PSOOA. On the contrary, a difference was found in contractility between eyes with SO overaction and control eyes, which may indicate abnormal innervation resulting in increased muscle stimulation. The excess innervation may primarily act on the posterior half of SO, where the contractile changes mainly occurred, which is in accordance with the anatomy of SO (5). Later studies would be recommended to further illustrate oblique muscle and other EOMs cross-section, contractility, and pulley locations to get a better understanding of PSOOA and PIOOA, thus helping choose more appropriate operation method.Conclusions
In PSOOA, theAcknowledgements
The study was supported byReferences
1.
Clark RA, Demer JL. Enhanced vertical
2. Demer JL, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci 1995; 36: 906-913.
3. Jiang L, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior rectus muscle in superior oblique muscle palsy. Ophthalmology 2008; 115: 2079-2086.
4.
Kushner BJ. Multiple mechanisms of extraocular muscle
"
5.
Kushner BJ. Vertical Strabismus. In: Hoyt GS, Taylor D
(
Figures