0092
Dynamic Diffusion Tensor Imaging in Normal and Compartment Syndrome Calf Muscle with MEDITI1Radiology, NYU Langone Medical Center, New York, NY, United States, 2Center for Advanced Imaging and Innovation (CAIIR), New York, NY, United States, 3NYU Tandon School of Engineering
Synopsis
We describe measurement of skeletal muscle kinematics with a multiple echo diffusion tensor imaging (MEDITI) in clinical scanners. This approach allows characterization of the microstructural dynamics in healthy and diseased muscle. Combining the accelerated MEDITI directional encoding with a radial k-space trajectory and compressed sensing reconstruction allows spatially resolved DTI with a continuous temporal resolution of 16 s. Using an MR-compatible ergometer, post-exercise recovery of DTI metrics in calf muscle were quantified in a pilot cohort of 2 volunteers and 4 subjects with chronic exertional compartment syndrome (CECS). Results indicate anisotropic exercise response and recovery with kinetics differing from relaxation contrast.
Purpose
Diffusion tensor imaging (DTI) of skeletal muscle can be applied to probe myofiber architecture at rest, with exertion, and in pathology. Exercise is a key challenge that has been widely employed with other MR contrasts1-4 with continuous temporal monitoring, while for diffusion is often applied coarsely5-7. Recently, post-exercise dynamic diffusion MRI has gained more attention7,8 but conventional singly-encoded sequences can limit temporal resolution. Another method (MEDITATE) for compressing the required directional encodings has been demonstrated using multiple echoes9. A single-voxel variant (SV-MEDITATE) has been applied to measure dynamic anisotropic diffusion exercise response in normal calf muscles10 and thigh muscles in dermatomyositis11. A spatially-resolved variant (MEDITI) with a multi-spoke Single Trajectory Radial (STAR) k-space trajectory12 and compressed sensing reconstruction, has shown dynamic DTI of a rotating phantom and calf muscle13. We evaluate MEDITI in the calf following in-scanner exercise in normal controls and chronic exertional compartment syndrome (CECS) patients.Methods
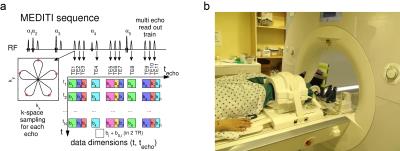
In this HIPAA-compliant, IRB-approved study, subjects provided written informed consent for a unilateral calf MRI in a Siemens Skyra 3 T scanner (1 volunteer, 4 CECS patients) or Prisma 3 T scanner (1 volunteer) and 15-channel knee coil. Subjects were positioned in an MR-compatible plantarflexion ergometer14, provided with elastic band resistance (Fig. 1) and performed repeated plantarflexion for 3 minutes. The MEDITI pulse sequence (Fig. 1) captures 11 echoes with a 5-petal STAR-trajectory. The angle between consecutive STAR trajectories is chosen according to the GRASP-scheme (Golden Radio Angle Radial Sparse Parallel). Other parameters: (TE: 90-245 ms, isotropic b-values: 167-790 s/mm2, flip angles 61°/73°/85°/45°/85°, TR=2000ms, 3x3x10 mm3 resolution). A series of unweighted (S0) and weighted (S) images at time-points before, during and after exercise were generated (total durations 21-34 min). Phase maps from low resolution reconstructions of each individual echo k-space removed intershot phase errors prior to dynamic reconstruction. Readouts from 4 consecutive TRs with the same diffusion weighting were combined for each frame, giving temporal resolution of 16 s. Sparsifying transforms exploit the similarity between diffusion weighted images along the echo (techo)- and the time (t)-dimensions to avoid undersampling artifacts:
$$\hat X = \arg {\min _X}\left\{ {\left\| {E \cdot X - Y} \right\|_2^2 + {\lambda _{PCA}}{{\left\| {PC{A^{{t_{echo}}}}X} \right\|}_1} + {\lambda _{PCAt}}{{\left\| {PC{A^t}X} \right\|}_1} + {\lambda _{TV}}{{\left\| {T{V^{xy}}X} \right\|}_1}} \right\}$$
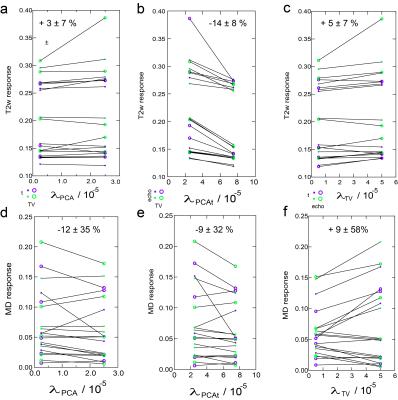
with X the time-series of images to be reconstructed, Y its k-space, E the multicoil encoding matrix, including coil sensitivities15 and the NuFFT-transform, PCA the Principal Component Analysis transform, TVxy the in plane total variation transform and λPCA, λPCAt and λTV regularization parameters chosen heuristically as 2.5e-5, 2.5e-5 and 5e-5 multiplied by the norm of X. Solutions were found using a non-linear conjugate gradient method16. Time points during exercise, identified in a first reconstruction , were removed prior to final reconstruction. The diffusion tensor was estimated with a cylindrical model, generating mean diffusivity (MD), axial (λ1), and radial (λrad) diffusion maps. Normalizing the post-exercise period by the average pre-exercise map generated diffusion response maps, which were subjected to post-hoc temporal binomial smoothing (5 frame width). Muscle compartments (anterior tibialis, peroneous longus, soleus, and gastrocnemius) were segmented on low-diffusion weighted images and response curves derived. Each post-exercise period was fit to a monoexponential decay with baseline to estimate total response and decay rate. In 3 subjects, MEDITI series were reconstructed for 8 different sets of regularization parameters (λPCA, λPCAt ,λTV) to determine variability of kinematic metrics with reconstruction. Sensitivity to these parameters was considered for compartments with T2w response of at least +10%.Results
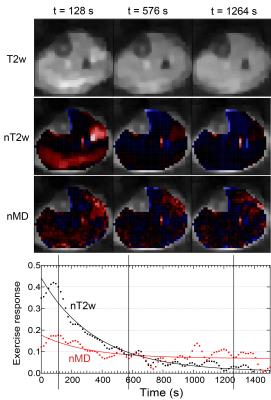
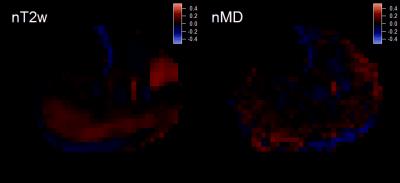
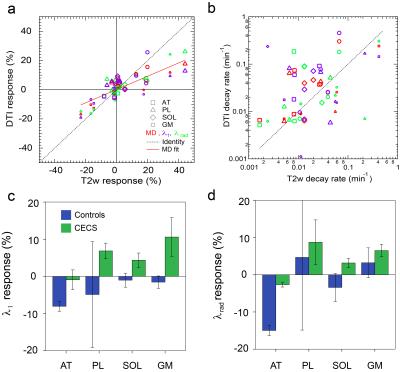
Figures 2 and 3 show example T2w images, T2w response maps, and MD response maps in one CECS subject. Response time dependences are also shown from the peroneus longus (PL). T2w response is larger and faster to recover than MD response. Figure 4 shows group responses and decay rates, showing correlation between T2w and diffusion metric response. Gastrocnemius medialis (GM) muscle showed strong response, with radial typically exceeding axial response. Diffusion responses in CECS patients were generally positive while controls trended negative. Figure 5 shows sensitivity of T2w and MD responses in activated muscles, in which the most universal trend is a reduction in T2w response with temporal regularization.Discussion
This work shows feasibility of MEDITI for dynamic DTI in controls and CECS patients with exercise. Response to exercise was observed primarily in GM and PL muscles, consistent with plantarflexion exercise, with significant correlation to response on T2w imaging but differing recovery kinetics of recovery. Preliminary tests indicate strongest sensitivity to temporal regularization. Future work will explore healthy and pathologic muscle behavior more comprehensively for reconstruction optimization and mapping of DTI muscle kinetics.Acknowledgements
This work was supported by the NIH (R21EB009435).References
1. Zhou H, Novotny JE. Cine phase contrast MRI to measure continuum Lagrangian finite strain fields in contracting skeletal muscle. Journal of Magnetic Resonance Imaging 2007;25(1):175-184.
2. Litwiller D, Amrami K, Dahm D, Smith J, Laskowski E, Stuart M, Felmlee J. Chronic exertional compartment syndrome of the lower extremities: improved screening using a novel dual birdcage coil and in-scanner exercise protocol. Skeletal Radiology 2007;36(11):1067-1075.
3. Bendahan D, Giannesini B, Cozzone PJ. Functional investigations of exercising muscle: a noninvasive magnetic resonance spectroscopy-magnetic resonance imaging approach. Cellular and Molecular Life Sciences (CMLS) 2004;61(9):1001-1015.
4. Andreisek G, White LM, Sussman MS, Langer DL, Patel C, Su JWS, Haider MA, Stainsby JA. T2*-Weighted and Arterial Spin Labeling MRI of Calf Muscles in Healthy Volunteers and Patients With Chronic Exertional Compartment Syndrome: Preliminary Experience. Am J Roentgenol 2009;193(4):W327-W333.
5. Ababneh ZQ, Ababneh R, Maier SE, Winalski CS, Oshio K, Ababneh AM, Mulkern RV. On the correlation between T(2) and tissue diffusion coefficients in exercised muscle: quantitative measurements at 3T within the tibialis anterior. MAGMA 2008;21(4):273-278.
6. Morvan D, Leroy-Willig A, Malgouyres A, Cuenod CA, Jehenson P, Syrota A. Simultaneous temperature and regional blood volume measurements in human muscle using an MRI fast diffusion technique. Magn Reson Med 1993;29(3):371-377.
7. Filli L, Boss A, Wurnig MC, Kenkel D, Andreisek G, Guggenberger R. Dynamic intravoxel incoherent motion imaging of skeletal muscle at rest and after exercise. NMR Biomed 2015;28(2):240-246.
8. Rockel C, Akbari A, Kumbhare DA, Noseworthy MD. Dynamic DTI (dDTI) shows differing temporal activation patterns in post-exercise skeletal muscles. MAGMA 2016.
9. Baete SH, Cho G, Sigmund EE. Multiple-echo diffusion tensor acquisition technique (MEDITATE) on a 3T clinical scanner. NMR Biomed 2013;26(11):1471-1483.
10. Baete SH, Cho GY, Sigmund EE. Dynamic diffusion-tensor measurements in muscle tissue using the single-line multiple-echo diffusion-tensor acquisition technique at 3T. NMR Biomed 2015;28(6):667-678.
11. Sigmund EE, Baete SH, Luo T, Patel K, Bruno M, Mossa D, Stoffel D, Femia A, Ramachandran S, Franks A, Bencardino J. Assessment of thigh muscle in healthy controls and dermatomyositis patients with diffusion tensor imaging, intravoxel incoherent motion, and dynamical DTI. Proceedings of International Socieity of Magnetic Resonance in Medicine 2015; Toronto.
12. Sarty GE. Single TrAjectory radial (STAR) imaging. Magn Reson Med 2004;51(3):445-451.
13. Baete S, Raya JG, Knoll F, Cho GY, Parasoglou P, Brown R, Block KT, Otazo R, Bencardino J. Feasibility of In Vivo Dynamic Diffusion Tensor Imaging on a 3T clinical scanner with a Multi Echo Sequence and compressed sensing reconstruction. Proceedings of International Society of Magnetic Resonance in Medicine 2015; Toronto.
14. Che X, Brown R, Alon L, Regatte RR, Parasoglou P. A Low-Cost MR Compatible Ergometer For Assessing Lower Leg Muscle Metabolism. Proceedings of International Society of Magnetic Resonance in Medicine 2016; Singapore.
15. Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med 2010;64(3):767-776.
16. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58(6):1182-1195.
Figures