0089
13C/31P MRS biomarkers of disease progression and response to gene therapy in a mouse model of Pompe disease1Department of Radiology, Leiden University Medical Center, C.J. Gorter Center for High-field MRI, Leiden, Netherlands, 2Department of Pediatrics, University of Florida, Gainesville, FL, United States, 3Department of Genetics, Stanford School of Medicine, Stanford, CA, United States, 4Department of Radiology, University of Washington, Seattle, WA, United States, 5Department of Physiology and Functional Genomics, University of Florida, Gainesville, FL, United States
Synopsis
With the emergence of rAAV-based gene therapy clinical trials in patients with glycogen storage disorders such as Pompe disease, there is a pressing need for early and non-invasive markers to assess treatment efficacy. While 13C-MRS has been used for detection of glycogen in muscle, its clinical implementation remains limited, due to its low natural abundance and inherent low sensitivity. 31P-MRS has higher sensitivity and can probe intermediates of glucose/glycogen metabolism. We sought to identify new biomarkers of Pompe disease progression in muscle using 13C/31P-MRS and 1H-HR-MAS in the mouse model of the disease, and tested their sensitivity to rAAV therapy.
INTRODUCTION
Pompe disease is a rapidly progressive degenerative neuromuscular condition characterized by a deficiency in alpha-glucosidase (GAA), the enzyme responsible for lysosomal glycogen breakdown. Although enzyme replacement therapy has been successful in improving survival rate there is still no cure to the disease. An alternative strategy is gene therapy, which has the potential to correct gene expression with a single administration of recombinant adeno-associated virus (rAAV). Preclinical and clinical investigation of all mechanistic aspects of the disease and treatment efficacy require appropriate non-invasive biomarkers, which are currently lacking. In this work we used a combination of high field (11.1T) 13C/31P MR spectroscopy (MRS) in vivo and tissue 1H high-resolution magic-angle spinning NMR (1H-HR-MAS) in the alpha-glucosidase knock out mouse (Gaa-/-) to 1) identify new biomarkers of metabolic disturbances over the course of the disease; 2) assess the sensitivity of these markers to rAAV2/9-desmin-GAA treatment in skeletal muscle.METHODS
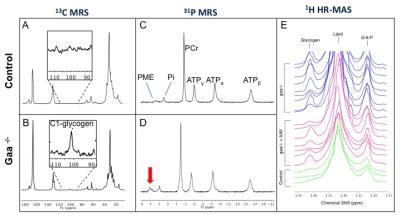
In vivo 31P and 13C-MRS data were acquired at 11.1T (Agilent, Inc., VnmrJ2.3) using 5x8mm2 surface coils (31P or 13C) under the calf of the animal, and a 1.5cm diameter 1H coil perpendicular for shimming. 31P-MRS were acquired with a hard pulse (20μs), TR=1s, 1200 averages, 4096 points, and BW=10k. After phase and baseline correction in MestReNova 10.0, PCr frequency was set to 0 ppm and phosphomonoester (PME), inorganic phosphate (Pi), PCr, γ-ATP, α-ATP, and β-ATP peaks were integrated. Results are presented as relative metabolites signals. 13C–MRS were acquired with a hard pulse (7.2μs), TR=500ms, 7200 averages and 1H-WALTZ-16 decoupling. Results are expressed as C1-glycogen (100.5 ppm) signal-to-noise ratio. Longitudinal changes: 31P-MRS was performed in Gaa-/- mice and wild-type at 2 (n=6), 6 (n=4), 12 (n=7), and 18 month-old (n=5). rAAV2/9-desmin-GAA study: 2mo Gaa-/- mice received unilateral rAAV2/9-desmin-GAA injections (5x1010vg) in the gastrocnemius and tibialis anterior muscles. Treated (Gaa-/-+rAAV, n=8), untreated (Gaa-/-, n=8) and wild type legs (Control, n=4) were scanned 28 days after treatment. Muscles were collected for correlative studies. 1H-HR-MAS was acquired using a 4mm probe at 600 MHz (Bruker; Topspin 3.2). Gastrocnemius tissue samples (25+/-2mg) from control (n=3), Gaa-/- (n=3) and Gaa-/-+rAAV (n=6) were prepared as previously described1 and 1D-NOESY with presaturation was acquired (128 scans, sw= 10 ppm, 16k points, D1=2s, Tm=90ms). Processing included exponential window function, 0.5Hz line broadening, zero-filling, and phase and baseline correction. Metabolites were quantified using MestReNova Simple Mixture Analysis (SMA) plugin and normalized to the total signal. GAA activity and glycogen concentration were assessed as described previously.2 Statistical analyses were done in GraphPad Prism using one-way ANOVA and Holm-Sidak’s multiple comparison tests. Adjusted p-values are reported.RESULTS
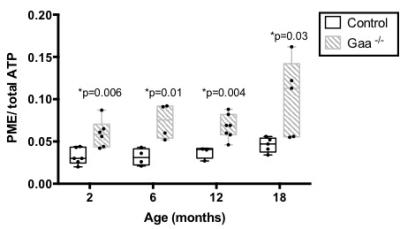
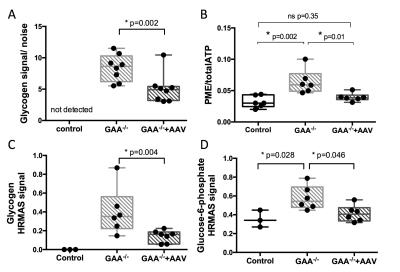
1) PME/ATPtotal and G-6-P are elevated in Gaa-/- mice. In vivo 31P-MRS showed no significant difference in PCr/ATP, Pi/(Pi+PCr), or pH between Gaa-/- and controls at 2, 6, 12, and 18 months. However, as early as 2 month-old, we observed a significantly higher PME/ATPtotal in Gaa-/- compared to control (p-values ranging from 0.03 to 0.009) (Figure 1C,D and Figure 2). In addition, HR-MAS revealed higher G-6-P and glycogen levels in the gastrocnemius of Gaa-/- as compared to controls (Figure 3C,D).
2) Glycogen, PME/ATPtotal and G-6-P levels are corrected by rAAV2/9-desmin-GAA injection in Gaa-/-. rAAV successfully restored high levels of GAA activity. C1-glycogen was detected in vivo in Gaa-/-. However, despite 1H-decoupling and high field strength, it was not reliably detected in controls within a reasonable acquisition time (1hr), due to low glycogen concentration in healthy muscles (Figure 1A,B). In Gaa-/-, glycogen signal-to-noise and PME/ATPtotal were significantly lower in the treated leg as compared to the untreated leg (Figure 2). GAA activity between 10-15 nmol/hr/mg was sufficient to significantly decrease all glycogen measures in Gaa-/-+rAAV animals, and higher activity did not induce further glycogen clearance. Glycogen and G-6-P as measured by HR-MAS were also significantly reduced after treatment (Figure 3C,D).
DISCUSSION
We identified PME as a new and sensitive 31P-MRS biomarker of disease progression and response to gene therapy in Gaa-/- mice. rAAV treatment not only restored normal levels of GAA activity and induced glycogen clearance but also significantly decreased the PME signal. Elevated PME levels have previously been observed in patients with glycogen storage disorder type III.3 Our HR-MAS results suggest that the accumulation of G-6-P may greatly contribute in the higher in vivo PME signal. This is consistent with recent work in Gaa-/- mice reporting high G-6-P concentration and increased glycogen synthase activity.4 Since 31P-MRS has a higher sensitivity than 13C, this approach has potential for clinical translation as a companion biomarker, in particular in the study of adjuvant therapy targeting glycogen metabolism.Acknowledgements
This work was funded by the Muscular Dystrophy Association research grant MDA218585 to C.B. and the National High Magnetic Field Laboratory.References
1. Beckonert, O., et al. (2010). High-resolution magic-angle-spinning NMR spectroscopy for metabolic profiling of intact tissues, 5(6), 1019–1032. http://doi.org/10.1038/nprot.2010.45
2. Todd AG, McElroy JA, Grange RW, Fuller DD, Walter GA, Byrne BJ, Falk DJ. Correcting Neuromuscular Deficits With Gene Therapy in Pompe Disease. Ann Neurol. 2015 Aug;78(2):222-34. doi: 10.1002/ana.24433.
3. Wary C, , et al. 2010. Investigating glycogenosis type III patients with multi-parametric functional NMR imaging and spectroscopy. Neuromuscular disorders NMD 20, no. 8: 548-558.
4. Taylor KM, et al. Dysregulation of Multiple Facets of Glycogen Metabolism in a Murine Model of Pompe Disease. Müller M, ed. PLoS ONE. 2013;8(2):e56181. doi:10.1371/journal.pone.0056181.
Figures