0072
Variable Flip Angle Radial Turbo Spin Echo Technique for Abdominal T2 Mapping1Electrical and Computer Engineering, University of Arizona, Tucson, AZ, United States, 2Medical Imaging, University of Arizona, Tucson, AZ, United States
Synopsis
The estimation of T2 relaxation times within lesions can provide a quantitative method of classifying abdominal neoplasms. Accelerated T2 mapping approaches have been proposed using the Radial TSE (RADTSE) sequence, where high resolution images at multiple TEs are reconstructed from data acquired in a single breath hold. However, the slice coverage for TSE based breath-held imaging is SAR restricted, motivating the need to reduce the refocusing flip angle. We present a variable refocusing flip angle RADTSE sequence designed to optimize the signal evolution for T2 mapping in the abdomen with reduced SAR, thereby increasing the slice coverage.
Introduction
Turbo spin-echo (TSE) techniques are used routinely in the clinic for abdominal imaging. One of the main applications is the characterization of focal liver lesions. It was shown that quantification of the T2 relaxation time more accurately classifies liver lesions1-3. Traditional multi-echo spin-echo or fast spin-echo based T2 estimation suffers from prohibitively long scan times and are unsuitable for abdomen breath-hold imaging. Accelerated T2 mapping approaches have been proposed using the Radial TSE (RADTSE) sequence4,5 where TE images with high spatial and temporal resolution are reconstructed from data acquired in a single breath hold. Recently, an approach was presented6 to efficiently characterize focal liver lesions using RADTSE. One drawback of TSE-based breath-held pulse sequences is that the slice coverage is SAR restricted, which requires a reduction of the refocusing flip angles. Reduced flip angle schemes cause the signal in the latter echoes of long echo trains to fall below the noise level making them unusable for T2 estimation. In this work we present a variable refocusing flip angle (varFA) RADTSE technique designed for improving T2 mapping in the abdomen by optimizing the signal evolution. The varFA scheme also significantly reduces SAR thus, increasing slice efficiency compared to the constant refocusing angle counterpart.Variable flip angle design
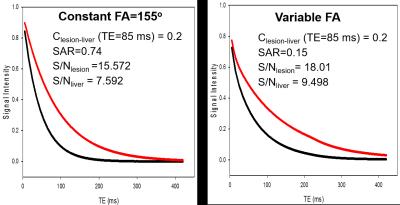
The varFA scheme was designed based on 3 parameters, the initial, center, and minimum flip angle7. The parameters were optimized for the tasks of (1) improving the relative contrast between malignant lesions and liver, (2) maintaining the signal above the noise floor (S/N) through the echo train length (ETL), and (3) reducing SAR. S/N was measured by the area under the T2 decay curve, generated for the selected flip angle train using the extended phase graph (EPG) model8. SAR was approximated by the sum of the squared flip angles. The optimization was done in two steps: first, a set of candidate angles were chosen to maximize S/N for a desired lesion-to-liver contrast ratio. Then, a Monte Carlo simulation was performed to determine the set of angles that minimized the T2 estimation error while also reducing SAR. Figure 1 shows a plot of T2 decay curves corresponding to lesion (T2=80 ms) and liver (T2=40 ms) for the constant and variable refocusing flip angle schemes. The varFA scheme maintains the lesion-to-liver contrast, typically seen in the clinic at TE=85ms with a constant flip angle train, while S/N is increased for both liver and lesion. SAR for the varFA approach is ~5x lower compared to the constant flip angle scheme.Methods
The varFA-RADTSE pulse sequence was implemented on the Siemens platform. The efficiency of T2 estimation in the presence of variable refocusing flip angles was evaluated using gel phantoms with T2s corresponding to liver and lesion tissue types. Data with varFA-RADTSE were acquired with ETL of 32 and 64, echo spacing=8msec, 192 radial views. Reference T2 values were estimated using a single-echo spin-echo (SE) method acquiring data at 16 TEs in increments of 8 msec. Patient data were acquired at 1.5T, with the constant and varFA RADTSE with ETL=32 and 64, respectively, echo spacing=6.2msec, and 192 radial views.
Composite anatomical images were reconstructed from all 192 radial views using a regridding algorithm. Individual TE images (32 or 64 depending on ETL) were reconstructed from undersampled RADTSE data (i.e., 6 or 9 views per TE depending on ETL) using the iterative model-based algorithm proposed in4. A pre-computed dictionary based on the forward signal model9 was used to estimate the T2 maps.
Results and Discussions
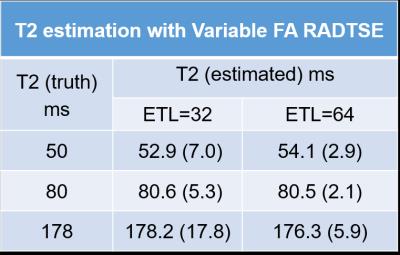
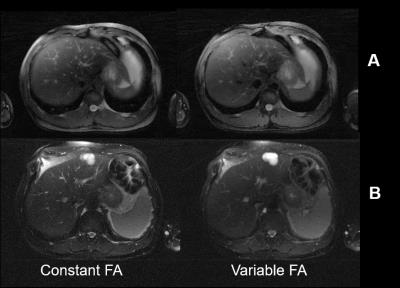
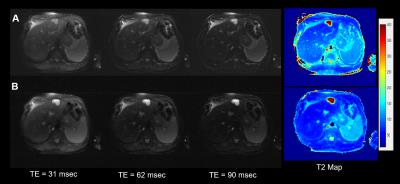
Phantom T2 values estimated from varFA-RADTSE with ETL=32 and 64 are compared to the SE reference in Figure 2. Note that the T2 values match the SE reference. Figure 3 shows the composite images using the constant and variable flip angle RADTSE sequences for a normal volunteer (Fig.2A) and a subject with a liver lesion (Fig.2B). Images reconstructed at different TEs and the corresponding T2 map are shown in Figure 4 for the two sequences. It can be observed that varFA-RADTSE provides images with comparable contrast as the constant flip angle case even though the ETL is twice as long. While the estimated T2 values fall in a similar range, the constant flip angle T2 map appears noisier. The varFA-RADTSE provided better slice coverage (13 slices per 18sec breath-hold), whereas, the constant flip angle RADTSE was restricted to 7 slices due to SAR.Conclusion
A variable flip angle radial TSE sequence is presented to improve the slice coverage for breath-held abdomen T2 mapping. The flip angles are optimized for efficient T2 estimation while maintaining minimal SAR at longer echo train lengths.Acknowledgements
Arizona Biomedical Research Commission (ADHS14-082996)References
1. Farraher SW, Jara H, Chang KJ, Ozonoff A, Soto JA: Differentiation of hepatocellular carcinoma and hepatic metastasis from cysts and hemangiomas with calculated T2 relaxation times and the T1/T2 relaxation times ratio. Journal of Magnetic Resonance Imaging 2006, 24(6):1333-1341.
2. Altbach M: A radial magnetic resonance imaging method for imaging abdominal neoplasms. In: Methods of Cancer Diagnosis, Therapy, and Prognosis. vol. 5: Springer, Netherlands; 2009: 21-32.
3. Kim YH, Saini S, Blake MA, Harisinghani M, Chiou YY, Lee WJ, Yu JS, Hahn PF: Distinguishing hepatic metastases from hemangiomas: qualitative and quantitative diagnostic performance through dual echo respiratory-triggered fast spin echo magnetic resonance imaging. Journal of computer assisted tomography 2005, 29(5):571-579.
4. Huang C., Bilgin A., Barr T., Altbach M, T2 relaxometry with indirect echo compensation from highly undersampled data, Magnetic Resonance in Medicine, 70(4) 2013
5. Altbach M., Bilgin A., Li Z., Clarkson E., Trouard T., Gmitro A., Processing of radial fast spin-echo data for obtaining T2 estimates from a single k-space data set, Magnetic Resonance in Medicine, 54 2005
6. Keerthivasan MB, L Jeffries, D Blew, JP Galons, P Sharma, Bilgin A, Martin DR, Altbach MI. Fast reconstruction of T2 maps with indirect echo compensation using highly undersampled radial Fast Spin Echo data. In Proceedings of ISMRM, Vol. 24, Singapore, 2016.
7. Busse RF, Brau ACS, Vu A, Michelich CR, Bayram E, Kijowski R, Reeder SB, Rowley HA. Effects of refocusing flip angle modulation and view ordering in 3D fast spin echo. Magn Reson Med 2008;60: 640–649
8. Hennig, Jürgen. "Echoes—how to generate, recognize, use or avoid them in MR-imaging sequences. Part I: Fundamental and not so fundamental properties of spin echoes." Concepts in Magnetic Resonance 3.3 (1991): 125-143.
9. Huang C., Altbach M, Fakhri G., Pattern recognition for rapid T2 mapping with stimulated echo compensation, Magnetic Resonance Imaging, 32, 2014
Figures