0069
Accelerating High Resolution Hyperpolarized 13C T2 Mapping Using a Local Low Rank plus Sparse Reconstruction1Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 2UC Berkeley-UCSF Graduate Program in Bioengineering, UCSF and University of California, Berkeley, San Francisco, CA, United States, 3HeartVista Inc., Los Altos, CA, United States
Synopsis
Hyperpolarized 13C probe development has allowed in vivo monitoring of different physiological processes relating to various diseases, including cancer and diabetes. Each new probe is typically characterized with polarization and T1 measurements, but T2 is also an important parameter for optimal sequence design, including progressive flip angle schemes. To improve the spatiotemporal resolution of T2 mapping sequences and subsequent multi-exponential analysis, this project investigated using a local low rank plus sparse reconstruction for 2-fold acceleration of in vivo T2 mapping with the bSSFP sequence.
Purpose
New advances in probe development for hyperpolarized (HP) 13C imaging has enabled monitoring of various physiological processes, such as metabolism and perfusion, of a variety of diseases.1,2 With each new developed probe, several measures are typically performed for the proper characterization of the probe to determine potential in vivo use, with the primary measures being T1 and polarization.3 T1 is important for proper sequence design due to the effects on achievable spatial and temporal resolution, as well as optimization of progressive flip angle schemes, and has been well studied for many current preclinical and clinical probes.2,3 The T2 values of hyperpolarized probes have recently also been investigated, especially in vivo, as they often are a critical component of optimal sequence design.4–7 T2 mapping using the balanced steady-state free precession (bSSFP) sequence has provided high spatial resolution maps for multi-exponential fitting of several compounds, including [13C]urea, [13C,15N2]urea, [2-13C]pyruvate, and [1-13C]lactate.5,8 However, T2 mapping acquisitions are slow and would benefit from acceleration methods such as compressed sensing9 to provide improvements in both spatial resolution and temporal resolution. In this study, we demonstrated 2-fold acceleration of in vivo bSSFP T2 mapping using a local low rank plus sparse (LLR+S) reconstruction10 that provided significant spatiotemporal improvements over existing methods.Methods
Retrospective simulations using LLR+S were performed by undersampling previously acquired [13C,15N2]urea and [1-13C]lactate datasets, which were acquired as 2D coronal projections with parameters listed in Reed et al.5 and Milshteyn et al.,8 respectively. For each time-point, a different 50% undersampling variable-density pattern was used, and the structural similarity index (SSIM) was used to assess the reconstruction as well as comparison of multi-exponential fits. The initial in vivo studies were performed with both a fully sampled acquisition and an accelerated acquisition (HP001 and [13C,15N2]urea). The parameters for the fully sampled acquisition were: 14x7 cm2 FOV, 140x70 matrix size, 1.6ms TBW 4 sinc pulse, 8.5-12.5ms TR, 4.25-6.25ms TE, 90°-180° θ/2-θ flip angles, 20 time-points, total scan time of 11.9-17.5s. The parameters for the HP001 (bis-1,1-(hydroxymethyl)-[1-13C]cyclopropane-d8) and [2-13C]pyruvate accelerated acquisitions were similar to the fully sampled acquisition with an 8.5/4.25ms TR/TE, but with 2-fold acceleration, leading to a total scan time of 5.95s. The parameters for the [13C,15N2]urea undersampled acquisition were: 14x7 cm2 FOV, 280x140 matrix size, 1.6ms TBW 4 sinc pulse, 15ms TR, 7.5ms TE, 90°-180° θ/2-θ flip angles, 20 time-points, total scan time of 11.9s. The resulting data was reconstructed using the LLR+S algorithm and fit using a nonnegative least squares algorithm, as described in Reed et al.6 The experiments were conducted on a 3T GE MR scanner and DNP experiments used a HyperSense polarizer. The scans started at 20s (HP001) or 30s ([13C,15N2]urea and [2-13C]pyruvate) after beginning of injection and 3mL of 100mM HP001, 80mM [2-13C]pyruvate, and 110mM [13C,15N2]urea was injected over 12s via tail vein catheters in three Sprague-Dawley rats.Results and Discussion
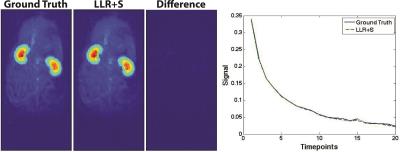
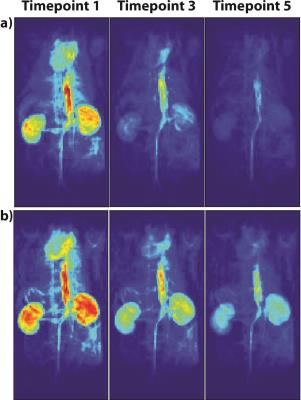
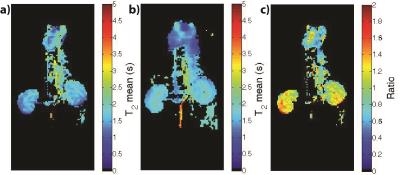
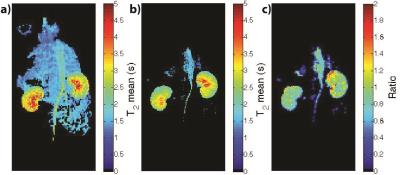
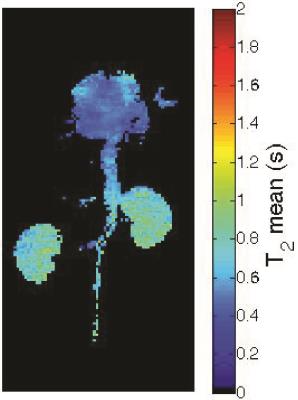
The LLR+S reconstruction of the retrospectively simulated data, which can be seen in Figure 1 for [13C,15N2]urea, was in good agreement with the fully sampled data in kidney and vasculature regions, based on the SSIM (urea: 0.992, lactate: 0.947). The prospective LLR+S reconstructed acquisitions also matched up well with the fully sampled acquisitions, which can be seen in three of the first five time-points of the HP001 acquisitions in Figure 2. The subsequent reconstructed T2 maps were also in good agreement as evidenced by the ratio maps of the fully sampled and accelerated acquisitions for HP001 (Figure 3) and [13C,15N2]urea (Figure 4), which had an average value of 1.12±0.22 and 0.94±0.21 in the kidneys, respectively. The urea acquisition also demonstrates the capability of sub-millimeter in-plane resolution acquisitions for HP probes with sufficient acceleration. Figure 5 shows the [2-13C]pyruvate acquisition T2 map with 1x1 mm2 in-plane resolution. The average value in the kidneys, 0.786±0.13, agreed well with previously acquired, lower resolution T2 maps.8 The LLR+S reconstruction allowed ~6-fold improvement in resolution even with [2-13C]pyruvate having a relatively short in vivo average T2.Conclusion
We demonstrated the ability to acquire undersampled T2 mapping data of various HP probes that correlated well with both fully sampled acquisitions and literature values. The LLR+S reconstruction allowed for improvements in spatiotemporal resolution, achieving 1x1 mm2 and sub-millimeter in-plane resolution. Further development of the method, such as increasing the amount of undersampling, may improve multi-exponential fitting via detection of short-lived components, potentially allow for 3D T2 mapping of hyperpolarized probes, and be translated to the clinic to optimize current HP acquisitions.Acknowledgements
The authors would like to thank Mark Van Criekinge, Lucas Carvajal, and Dr. Robert A. Bok for all their help and funding from the NIH (P41EB013598 and R01EB017449).References
1. Kurhanewicz, J. et al. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia 13, 81–97 (2011).
2. Brindle, K. M., Bohndiek, S. E., Gallagher, F. A. & Kettunen, M. I. Tumor Imaging Using Hyperpolarized Resonance Spectroscopy 13C Magnetic. Magn. Reson. Med. 66, 505–519 (2011).
3. Chaumeil, M. M., Najac, C. & Ronen, S. M. in Methods in Enzymology 561, (2015).
4. Yen, Y. F. et al. T2 relaxation times of 13C metabolites in a rat hepatocellular carcinoma model measured in vivo using 13C-MRS of hyperpolarized [1-13C]pyruvate. NMR Biomed. 23, 414–423 (2010).
5. Reed, G. D. et al. High Resolution 13C MRI With Hyperpolarized Urea: In Vivo T2 Mapping and 15N Labeling Effects. IEEE Trans. Med. Imaging 33, 362–371 (2014).
6. Reed, G. D. et al. Imaging Renal Urea Handling in Rats at Millimeter Resolution using Hyperpolarized Magnetic Resonance Relaxometry. Tomography 2, 125–137 (2016).
7. Shang, H. et al. Variable Flip Angle Design for Balanced SSFP Transient State Imaging to Improve HP 13C MRI. in Proceedings of the 23rd Annual Meeting of ISMRM, Toronto, Canada 3768 (2015).
8. Milshteyn, E. et al. Development of High Resolution 3D Hyperpolarized 13C Imaging Techniques. in Proceedings of the International Society for Magnetic Resonance in Medicine 23 (2015) 4599.
9. Feng, L. et al. Accelerated Cardiac T2 Mapping using Breath-hold Multiecho Fast Spin-Echo Pulse Sequence with k-t FOCUSS. Magn. Reson. Med. 65, 1661–1669 (2011).
10. Milshteyn, E. et al. Using a Low Rank plus Sparse Reconstruction Approach to Accelerate 3D Dynamic bSSFP Hyperpolarized Carbon-13 MR Imaging. in Proceedings of the International Society for Magnetic Resonance in Medicine 24 (2016) 2329.
Figures