0068
Single Breath-hold Abdominal T1 Mapping using 3-D Cartesian Sampling and Spatiotemporally Constrained Reconstruction1Pattern Recognition Lab, Department of Computer Science, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2MR Applications Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Volumetric T1 mapping in the abdomen is desirable for whole liver assessment of hepatic diseases. In case of breath-hold imaging, accurate but time-consuming methods that sample the relaxation curve (IR or Look-Locker) are restricted to few slices only. To address these limitations, sparse Cartesian sampling with spatiotemporal incoherence is utilized to render 3-D Look-Locker within a single breath-hold possible. We demonstrate feasibility in both phantom and in-vivo measurements. The proposed method shows high agreement with a 2-D reference acquisition and enables an accurate mapping for a wide T1 range, including very low values due to its high temporal resolution.
Introduction
Abdominal T1 mapping can help diagnosing and staging hepatic diseases such as liver cirrhosis1. Yet, accurate methods that are based on sampling the relaxation curve are usually limited to a few slices in case of breath-hold imaging. Common 3-D techniques are often based on a variable-flip-angle approach, which is B1 sensitive, even when complemented with an additional B1 mapping acquisition and correction2. Look-Locker sequences are considered more accurate albeit time-consuming, restricting volumetric Look-Locker to static imaging only3. Sparse sampling with incoherence in both space and time can alleviate this problem. To this end, an existing 3-D CINE sequence prototype4,5 was extended to support inversion pulses and FLASH contrast.
We investigate whether the increased signal of 3-D acquisitions in combination with a sparse spatiotemporally incoherent sampling of the relaxation curve in high temporal resolution can yield T1 values in a wide range with high accuracy. To our knowledge, this is the first application of whole-liver T1 mapping and 3-D Cartesian Look-Locker within a breath-hold. Experiments include both phantom and in-vivo measurements.
Materials and Methods
A Look-Locker T1-mapping scheme with continuous sampling after an initial inversion pulse was used. We utilized a multi-TI CINE protocol with an IR-FLASH sequence featuring adiabatic inversion4. Time points in the reconstruction were assigned to contrasts after inversion pulses. For sufficiently high spatiotemporal sampling density, k-space segmentation with multiple inversions and therebetween a wait-time for free relaxation was introduced.
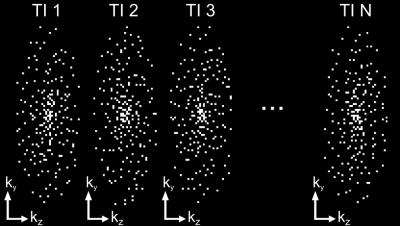
A variable-density spiral spokes pattern ensured Cartesian sampling with a high temporal resolution5 (~100ms), which allows to determine very low T1 values. For improved k-space coverage, multiple spiral arms were sampled in each shot and the set of spokes was rotated successively by the golden angle per shot and TI (Figure 1). Time-resolved reconstructions were performed using a FISTA algorithm6, which incorporates wavelet regularization in both space and time domain. 40 iterations with spatial/temporal regularization weights of 0.0006/0.007 were used. T1 maps were obtained using a phase-corrected multi-step parameter fitting utilizing a smoothed flip angle map.
A 3 T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) was used for all experiments. An 18-channel body coil was used for volunteers, a 20-channel head coil for phantom experiments. Reconstructions were compared against 2-D multi-slice reference measurements of a prototypical LL sequence by means of ROI mean and standard deviation:
In-vivo: axial slices from a 6-slice 2-D acquisition were compared against corresponding slices in 3-D
Phantom: assessment based on the T1–array of the NIST phantom7
3-D imaging parameters: FoV = 365x255x150mm3, matrix = 160x94x30, TR = 2.4ms, TE = 1ms, flip-angle = 6°, bandwidth = 1563Hz/Px, 19 TIs, ΔTI = 102ms, net acceleration = 15.4, acquisition window = 2s, wait time = 3.8s, 4 inversions.
2-D imaging parameters: FoV = 380x308mm2, matrix size = 192x125 (1mm2 interpolated), slice thickness = 5mm, TR = 3ms, TE = 1.3ms, flip-angle = 8°, bandwidth = 1530 Hz/px, 16 TIs, ΔTI = 225ms (2x acceleration), scan time = 22.8s (6 slices).
Results and Discussion
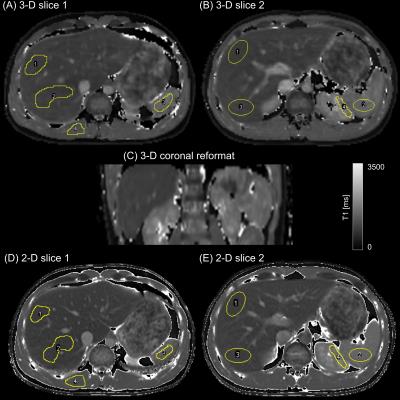
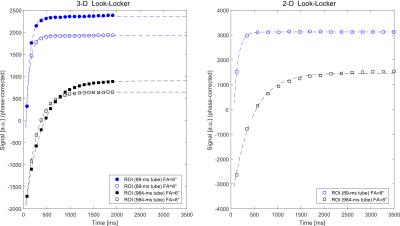
The 3-D+t image reconstructions took less than a minute using the scanner graphics hardware while T1 mapping on the CPU (not parallelized) required 5–10s per slice. In-vivo results in Figure 2 show axial slices and a coronal reformation from the 3-D acquisition (A-C) in comparison to the 2-D reference (D,E). Figure 3 illustrates the signal recovery of the 3-D and 2-D acquisition in comparison. Labeled ROIs used for the in-vivo quantitative evaluation, which is presented in Table 1, are shown. Table 2 summarizes the results of the phantom evaluation.
Average hepatic T1 values of 799±32 and 810±44ms between the 2-D reference and 3-D show very high agreement in vivo. The
phantom comparison shows excellent agreement with reference values for both methods
in a wide range. Yet, only the 3-D acquisition with its short ΔTI, allowed determining phantom tubes with T1
values as low as 60ms accurately.
While the visual appearance between 2-D and 3-D
is quite different due to image resolution, the 3-D acquisition, despite high
acceleration, is hardly affected by artifacts and allows delineating most
vessels and anatomical structures.
Conclusion
The feasibility of a 3-D Look-Locker acquisition for abdominal T1 mapping within a single breath-hold was demonstrated. Utilizing an efficient reconstruction framework for spatiotemporal sparsity, our method enables whole-liver mapping with a 2.3x2.3x5mm3 resolution in 23s. Excellent agreement with a 2-D reference was shown for volunteer and phantom data for a wide T1 range of 60–2000 ms. Additionally, the spatiotemporal sparsity enables the usage of very short ΔTIs making an accurate mapping of very low T1 values feasible. Future works aims at improving scan efficiency.Acknowledgements
This work was partly supported by the Research Training Group 1773 “Heterogeneous Image Systems”, funded by the German Research Foundation (DFG).References
[1] Haimerl M, Verloh N, Zeman F, et al. Assessment of Clinical Signs of Liver Cirrhosis Using T1 Mapping on Gd-EOB-DTPA-Enhanced 3T MRI. PLoS ONE. 2014;8(12):e85658
[2] Deoni S. High-resolution T1 mapping of the brain at 3T with driven equilibrium single pulse observation of T1 with high-speed incorporation of RF field inhomogeneities (DESPOT1-HIFI). J Magn Reson Imaging. 2007;26(4):1106-1111
[3] Henderson E, McKinnon G, Lee, TY, et al. A Fast 3D Look-Locker Method for Volumetric T1. Mapping Magn Reson Imaging 1999;17(8):1163-1171
[4] Stalder AF, Speier P, Zenge M, et al. Cardiac Multi-Contrast CINE: Real-Time Inversion-Recovery Balanced Steady-State Free Precession Imaging with Compressed-Sensing and Motion-Propagation. In: Proceedings of the 22nd Annual Meeting of ISMRM. 2014.#431
[5] Wetzl J, Lugauer F, Schmidt M, et al. Free-Breathing, Self-Navigated Isotropic 3-D CINE Imaging of the Whole Heart Using Cartesian Sampling. In: Proceedings of the 24th Annual Meeting of ISMRM. 2016.#411
[6] Liu J, Rapin J, Chang T, et al. Dynamic Cardiac MRI Reconstruction with Weighted Redundant Haar Wavelets. In: Proceedings of the 20th Annual Meeting of ISMRM. 2012.#178
[7] Keenan K, Stupic K, Boss M, et al. Multi-Site, Multi-Vendor Comparison of T1 Measurement Using ISMRM/NIST System Phantom. In: Proceedings of the 24th Annual Meeting of ISMRM. 2016.#3290
Figures