0062
Using diffusion MRI and tractography to identify macaque vertical occipital fasciculus1Center for Information and Neural Networks (CiNet), National Institute of Information and Communications Technology, Suita-shi, Japan, 2Graduate School of Frontier Biosciences, Osaka University, Suita-shi, Japan, 3Department of Psychological and Brain Sciences, Indiana University, Bloomington, IN, United States, 4Department of Psychology, Stanford University, Stanford, CA, United States, 5Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 6Department of Biomedical Science, University of Antwerp, Antwerp, Belgium, 7The Rockefeller University, New York, NY, United States, 8Neurophysiology Imaging Facility, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, National Eye Institute, National Institutes of Health, Bethesda, MD, United States
Synopsis
We evaluated the ability of diffusion MRI-based tractography to identify macaque vertical occipital fasciculus (VOF), an important but little-studied white-matter tract connecting dorsal and ventral visual cortex. We analyzed four macaque diffusion MRI datasets with different resolution. The high-resolution post-mortem dataset reliably detects the macaque VOF, in a consistent manner with previous invasive anatomical studies. Lower resolution in vivo data showed qualitatively consistent results, but the estimated tract endpoints are restricted to sulcus. Taken together, our results demonstrate that the need for high-resolution diffusion MRI to identify certain critical white matter tracts.
Purpose
The vertical occipital fasciculus (VOF, also termed Wernicke’s perpendicular fasciculus) was originally reported by Wernicke [1] in monkey as a white-matter tract connecting dorsal and ventral visual cortex; the tract was largely overlooked in the literarture [2]. Recently the homologous human VOF has attracted attention because diffusion MRI-based tractography studies reported this tract in relation to brain functions and health [3-5]. Given the significance of the VOF in human, there may be value in understanding its role in a primate model. This study evaluates the ability of diffusion MRI and tractography to identify the macaque VOF and compares the tractography VOF estimates with estimates from previous invasive studies.Methods
Dataset: The analyses are based on four macaque diffusion MRI datasets with different resolutions. M1 dataset was highest-resolution dataset acquired from a post-mortem rhesus macaque brain using Bruker 7T scanner at the National Institute of Health (250 μm isotropic, 121 directions, b = 4800 s/mm2 [6-7]). M2 dataset was acquired from a living rhesus macaque brain using a Bruker 4.7 T scanner at Max Planck Institute, Tübingen, Germany (750 μm isotropic, 61 directions, b = 1200 s/mm2). M3 dataset was acquired in a post-mortem rhesus macaque brain using a Bruker 4.7 T scanner at Max Planck Institute, Tübingen, Germany (800 μm isotropic, 61 directions, b = 4000 s/mm2 [8]). M4 dataset was was acquired from a living rhesus macaque brain using a 3T SIEMENS Trio MRI scanner at the Citigroup Biomedical Imaging Center of the Weill Cornell Medical College (1 mm isotropic, 64 directions, b = 2000 s/mm2).
Tractography method: We used Ensemble Tractography (ET [9]) to estimate the white matter tracts. We generated a candidate connectome using probabilistic tractography and five curvature thresholds (minimum radius of curvature, 0.25, 0.5, 1, 2, and 4 mm) in MRtrix [10]. We generated a total 2,500,000 streamlines for each macaque dataset. We used Linear Fascicle Evaluation (LiFE [11]) to remove the streamlines that make no significant contribution to explaining the diffusion measurements. Finally, macaque VOF was identified by using two coronal waypoint ROIs, located at approximately Z = 2 and Z = 11 in AC coordinate in D99 macaque brain atlas [12].
Results
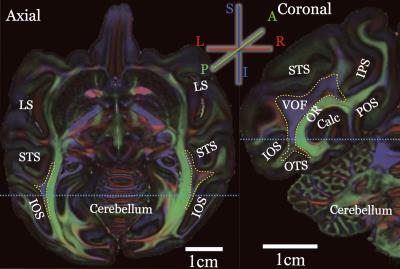
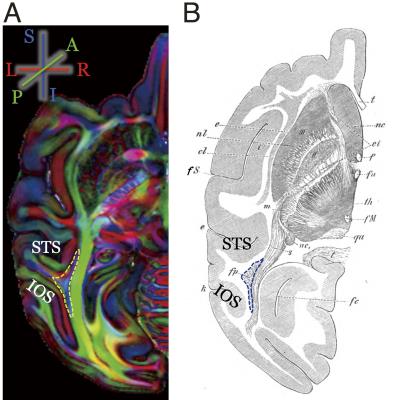
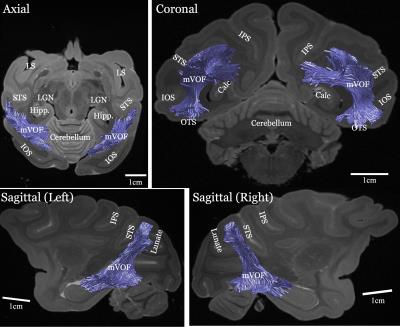
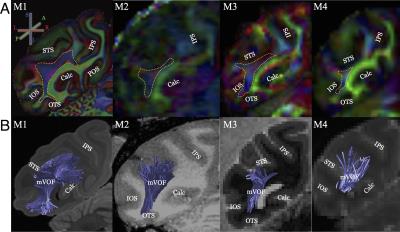
Figure 1 shows the Principal Diffusion Direction (PDD) map in the highest resolution ex vivo dataset (M1). The data in Figure 1 reveal macaque VOF with a superior-inferior diffusion direction (blue) in the lateral occipital white matter (outlined), communicating between dorsal and ventral visual cortex. The ventral portion of this tract is located between Inferior Occipital Sulcus (IOS) and the Superior Temporal Sulcus (STS; left panel, axial view, Figure 1). Figure 2 compares the PDD map of M1 with Wernicke’s classical study [1]. In Wernicke’s schematic, the VOF (“fp”) is surrounded by two sulci, which correspond to modern definitions of the STS and the IOS, consistent with M1. Figure 3 describes the trajectory of macaque VOF estimated by tractography. We evaluated the spatial proximity of the VOF endpoint with macaque brain atlas [13]. The dorsal VOF endpoints are near V3A, V4d, MT whereas ventral VOF endpoints are near V4v and TEO. This result is consistent with tract degeneration and tracer studies [14-16]. Finally, we compared the estimated VOF across datasets with different resolutions (Figure 4). The core of the VOF can be identified in all datasets. However, we found that the lower-resolution datasets miss cortical endpoints in the gyrus between IOS and OTS. Tractography based on high-resolution ex vivo data is significantly better for reconstructing the VOF compared to even high quality in vivo data.
Conclusion
We succeeded in identifying the macaque VOF using diffusion MRI-based tractography. At high-resolution the results of tractography are consistent with invasive macaque anatomical studies. This study demonstrates a specific advantage of ex vivo macaque diffusion MRI approach. In this application, it has a better ability to identify the tract compared to in vivo data with standard resolution (Figure 4), while it may still miss some tract endpoints [7].Acknowledgements
We thank Stelios Smirnakis for supporting in the collection of macaque diffusion MRI data, Kaoru Amano, Atsushi Wada and Noboru Nushi for providing the computer environment, Cesar F. Caiafa for providing analysis tools and Lee Michael Perry for technical assistance. This study is funded in part by JSPS Postdoctoral Fellowship for Research Abroad and the Grant-in-Aid for JSPS Research Fellow (to H.T.), Indiana University College of Arts and Sciences startup funds (to F.P.), Indiana Clinical and Translational Sciences Institute CTSI (GLUE Grant; supported by NIH grants ULTTR001108, ULTTR001106, ULTTR001107; to F.P.), Human Frontier Science Program Long-Term Fellowship (LT000418/2013-L, to J.S.), a Fondation pour la Recherche Médicale Post-doctoral fellowship (to J.S.), a Women&Science Post-doctoral Fellowship (to J.S.), a Bettencourt-Schueller Foundation Young Researcher Award (to J.S.), the intramural Research Program of the National Institutes of Health (to F.Q.Y. and D.A.L.), a Pew Scholar Award in the Biomedical Sciences (to W.A.F.), The Esther A. & Joseph Klingenstein Fund (to W.A.F.), a McKnight Scholars Award (to W.A.F.), the New York Stem Cell Foundation (NYSCF-R-NI23, to W.A.F.), the National Eye Institute (R01 EY021594-01A1, to W.A.F.) and NSF BCS-1228397 (to B.A.W.). H.T. is a Superlative Postdoctoral Fellow of Japan Society for the Promotion of Science. S.M.L. is a Howard Hughes Medical Institute International Student Research fellow. W.A.F. is a New York Stem Cell Foundation-Robertson Investigator.References
1. Wernicke C. Lehrbuch Der Gehirnkrankheiten Für Aerzte Und Studirende. 1881: Kassel Theodor Fischer.
2. Yeatman JD, Weiner, KS, Pestilli, F, et al. The Vertical Occipital Fasciculus: A Century of Controversy Resolved by in Vivo Measurements. Proc Nat Acad Sci U S A 2014; 111: E5214–23.
3. Yeatman JD, Rauschecker AM, Wandell, BA. Anatomy of the Visual Word Form Area: Adjacent Cortical Circuits and Long-Range White Matter Connections. Brain Lang 2013; 125: 146–55.
4. Duan Y, Norcia AM, Yeatman JD, and Mezer A. The Structural Properties of Major White Matter Tracts in Strabismic Amblyopia. Invest Ophthalmol Vis Sci 2015; 56(9): 5152–60.
5. Takemura H, Rokem A, Winawer J, et al. A Major Human White-Matter Pathway between Dorsal and Ventral Visual Cortex. Cereb Cortex 2016; 26(5): 2205–14.
6. Thomas C, Ye FQ, Irfanoglu MO, et al. Anatomical Accuracy Proceedings of the National Academy of Sciences of the United States of Americaof Brain Connections Derived from Diffusion MRI Tractography Is Inherently Limited. Proc Nat Acad Sci U S A 2014; 111: 16574-16579.
7. Reveley C, Seth AK, Pierpaoli C, et al. Superficial White Matter Fiber Systems Impede Detection of Long-Range Cortical Connections in Diffusion MR Tractography. Proc Nat Acad Sci U S A 2015; 112: E2820–2828.
8. Iturria-Medina, YA, Perez Fernandez DM, Morris, EJ, et al. Brain Hemispheric Structural Efficiency and Interconnectivity Rightward Asymmetry in Human and Nonhuman Primates. Cereb Cortex 2011; 21: 56–67.
9. Takemura H, Caiafa CF, Wandell BA, Pestilli, F. Ensemble Tractography. PLoS Comput Biol 2016; 12(2): e1004692.
10. Tournier JD, Calamante F, Connelly, A. MRtrix: Diffusion Tractography in Crossing Fiber Regions. International Journal of Imaging Systems and Technology 2012; 22: 53–66.
11. Pestilli F, Yeatman JD, Rokem A, et al. Evaluation and Statistical Inference for Human Connectomes. Nature Methods 2014; 11: 1058–63.
12. Reveley C, Gruslys A, Ye FQ, et al. Three-dimensional digital template atlas of the macaque brain. Cereb Cortex 2016; Epub ahead of print.
13. Saleem KS, Logothetis, NK. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates. 2012: San Diego: Academic Press.
14. Petr R, Holden LB, Jirout J. The efferent intercortical connections of the superficial cortex of the temporal lobe (macaca mulatta)*. J Neuropathol Exp Neurol. 1949; 8:100.
15. Schmahmann JD, Pandya D. Fiber Pathways of the Brain. 2006; New York: Oxford Univ Press.
16. Ungerleider LG, Galkin TW, Desimone R, Gattass R. Cortical Connections of Area V4 in the Macaque. Cerebral Cortex 2008; 18: 477–99.
Figures