0058
Mesh-based anatomically-constrained tractography for effective tracking termination and structural connectome construction1The Florey Institute of Neuroscience and Mental Health, Melbourne, Australia, 2The Florey Department of Neuroscience, University of Melbourne, Melbourne, Australia
Synopsis
This study introduces a novel diffusion MRI streamlines tractography framework called mesh-based anatomically-constrained tractography (MACT) that incorporates high-resolution surface models of various brain tissues as more accurate anatomical constraints in the fibre-tracking process. By detecting intersections between streamlines and tissue surfaces, MACT can effectively provide meaningful track terminations and inter-areal connections by associating streamlines with the structural labels of the intersected surfaces. This therefore minimises uncertainties caused by heuristic mechanisms of assigning streamlines to labelled structures in common image-based approaches. Methods that investigate the tractogram-based structural connectivity should benefit from the improved connectome reconstruction using the proposed technique.
Purpose
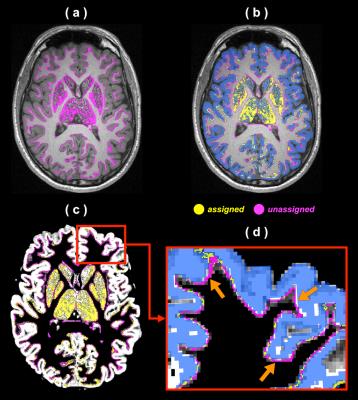
Anatomically-constrained tractography (ACT1) and similar variants2 can improve the biological plausibility of streamlines tractograms by using anatomical information to control termination of fibre tracking. These techniques typically use the tissue partial volume maps (PVMs) of T1-based brain segmentation as anatomical constraints to ensure that streamlines terminate within either cortical grey matter (CGM), sub-cortical grey matter (SGM), or brainstem. Streamline endpoints following these methods are often determined by the grey matter (GM) PVM, which can however be inconsistent with the brain parcellation image (see Figure 1). Such spatial mismatch can significantly limit the number of identifiable pairwise connections in the process of structural connectome construction3, and therefore additional node assignment mechanisms are required to compensate for such discrepancy3,4. To address this problem, this study proposes a novel mesh-based ACT (abbreviated to MACT) technique that incorporates more accurate anatomical constraints using high-resolution surface representations of brain tissues. Differing from the PVM-based ACT, MACT incorporates multiple tissue surface models into the tractography process and directly identifies intersections between streamlines and surfaces; thus, streamlines are not only terminated with meaningful endpoints but also effectively acquire structural labels defined on the tissue surfaces. This may avoid potential ambiguities induced by assigning streamlines to brain parcellations in the common image-based methods and should therefore be advantageous to structural connectome construction.Methods
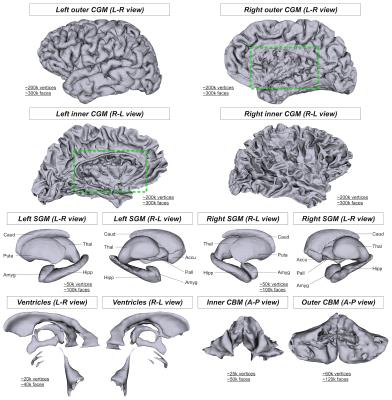
Tissue surface models: An in-house MATLAB (MathWorks) script was used to generate tissue surface models that serve as anatomical constraints in MACT. In this study, four types of brain tissue surfaces were created from structural T1s (0.9-mm isotropic resolution) acquired on a Siemens 3T MRI scanner with the following steps:
(a) CGM: Brainder5 was employed to crop the closed CGM surfaces generated by FreeSurfer6 in order to allow inter-hemisphere connections as well as connections to brainstem and cerebellum.
(b) SGM: The SGM surfaces obtained from FSL's FIRST7 were combined together.
(c) Cerebellum (CBM): FreeSurfer's surface tessellation and smoothing functions were applied to convert CBM labels to surfaces.
(d) Ventricles (or CSF): The surface meshes of the ventricles created from FreeSurfer's parcellation image were merged together.
(e) All of the above-mentioned tissue surfaces were aligned to the scanner coordinate.
Surface seeding: The mechanism of homogeneously seeding on the tissue surface is developed by taking the area of triangle (i.e. the mesh element) into account.
Fibre tracking: MACT follows the principle of ACT to accept or reject streamlines on the basis of both anatomical and tracking criteria1, where the main difference is that MACT detects possible intersecting tissue types at each fibre-tracking step. Three-dimensional surface lookup tables are developed to accelerate the processing speed. MACT is integrated with the features of ACT1 including the back-tracking algorithm and the tailored anatomical priors for SGM.
Results
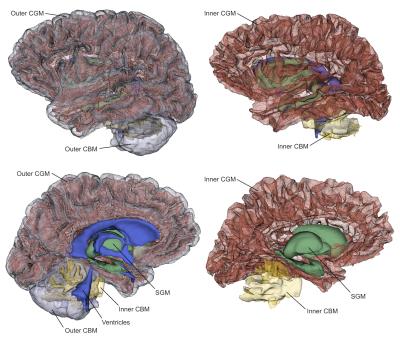
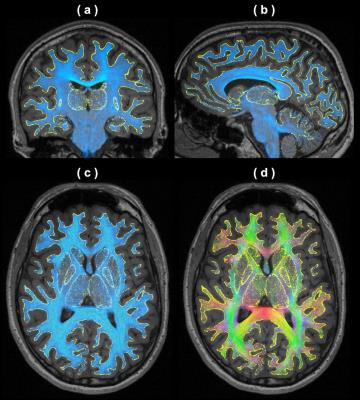
Figure 2 shows the outcomes of tissue surface preparation. Following the mesh processing, the inner and outer CGM surfaces became open surfaces; this enables, for instance, connections between brain hemispheres. Figure 3 demonstrates the aligned meshes that are used as anatomical constraints in MACT according to the tissue type. Figure 4 shows the results of MACT, in which fibre orientation distributions were computed from DWIs (2.5-mm cubic voxels, 60 directions, b=3000 s/mm2) using constrained spherical deconvolution8. Five million streamlines were generated using the iFOD2 algorithm9 with the surface seeding method. All the streamline endpoints can successfully identify structural label information defined on the surfaces.Discussion
Our data demonstrates that MACT is an effective technique for generating streamlines tractograms with meaningful track terminations determined by high-resolution surface meshes of brain tissues. The MACT framework enables using the same source anatomical information defined by various tissue surfaces at both the fibre-tracking and connectome construction stages, which can therefore minimise uncertainties induced by additional mechanisms commonly used in the image-based approaches for assigning streamlines to structural labels3. Also note that in the processing framework of the Human Connectome Project10, the tractogram map or direct cortical connectivity is obtained by only using the CGM surface to try to ensure that each streamline possesses at least two intersections. MACT has the advantage of incorporating more comprehensive surface models as anatomical constraints into the tractography process as well as compatibility with the emerging structure-informed tractogram filtering method for quantitative tractogram reconstruction11-13. Altogether, the proposed method should be advantageous to structural connectome construction.
Conclusion
We introduce a novel tractography framework that incorporates high-resolution surface models of brain tissues into the tractography process. Investigations of tractogram-based structural connectivity should benefit from the proposed method, which can also be combined with other neuroimaging modalities where the data is measured natively on the brain surface14.Acknowledgements
References
1. Smith RE, et al. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage. 2012; 62(3): 1924-38.
2. Girard G, et al. Towards quantitative connectivity analysis: reducing tractography biases. NeuroImage. 2014; 98: 266-78.
3. Yeh CH, et al. The influence of node assignment strategies and track termination criteria on diffusion MRI-based structural connectomics. Proc. ISMRM. 2016; p.118.
4. Smith et al. The effects of SIFT on the reproducibility and biological accuracy of the structural connectome. NeuroImage. 2015; 104: 253-65.
5. Winkler AM, et al. Measuring and comparing brain cortical surface area and other areal quantities. NeuroImage. 2012; 61(4): 1428-43.
6. Dale AM, et al. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999; 9: 179-94.
7. Patenaude B, et al. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011; 56(3): 907-22.
8. Tournier JD, et al. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. NeuroImage. 2007; 35(4): 1459-72.
9. Tournier JD, et al. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. Proc. ISMRM. 2010; p.1670.
10. Sotiropoulos SN, et al. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. NeuroImage. 2013; 80: 125-43.
11. Smith RE, et al. SIFT: Spherical-deconvolution informed filtering of tractograms. NeuroImage. 2013; 67: 298-312.
12. Daducci A, et al. Microstructure Informed Tractography: Pitfalls and Open Challenges. Front Neurosci. 2016; 10: 247.
13. Yeh CH, et al. Correction for diffusion MRI fibre tracking biases: The consequences for structural connectomic metrics. NeuroImage. 2016; 142: 150-62.
14. Glasser MF, et al. The Human Connectome Project's neuroimaging approach. Nat Neurosci. 2016; 19(9): 1175-87.
Figures