0057
A novel anatomy-based constrained global tractography1Université Paris-Saclay, Orsay, France, 2CEA/DRF/I2BM/NeuroSpin/UNIRS, Gif-sur-Yvette, France, 3CEA/DRF/I2BM/NeuroSpin/UNATI, Gif-sur-Yvette, France, 4http://cati-neuroimaging.com/, Orsay, France
Synopsis
Diffusion magnetic resonance imaging is still the unique tool capable of probe the structure of the brain connectivity in vivo. Although some great advances have been made in the past decade, reconstructed tractograms often lack of anatomically accuracy. The introduction of anatomical priors has become a promise land to tackle this issue, so in this work, we propose a general spin-glass-based global tractography framework constrained by anatomical priors to better represent the sharp turns of fibers entering a gyrus and connecting to the pial surface.
Purpose
Diffusion magnetic resonance imaging remains the unique technique available to probe in-vivo the human brain structural connectivity. The introduction of probabilistic [1,2] or global methods tackle the underlying ill-posed problem of creating more realistic numerical fibers. Those global approaches allow to keep a low curvature between connections [3,4,5,6] but tractograms still remain poorly anatomically reliable and some proposals have been made to increase the anatomical priors level [7,8,9]. In this work, we propose a global spin-glass-based tractography algorithm constraining anatomically the spins direction to embrace sharp turns when entering gyri and connecting to the cortex.
Methods
MRI dataset – One subject of the CONNECT/Archi database [12] was used. This
protocol includes a millimeter T1-weighted MPRAGE scan (TE=2.98ms/TR=2.3s), a HARDI dataset acquired at b=1500s/mm2 using 60 diffusion
directions (TE=117ms/TR=14s ) with a 1.7mm isotropic resolution and a
fieldmap to correct susceptibility artifacts. The T1-weighted dataset was processed using Freesurfer
[13] to infer the pial surface (81924 vertices).
The DWI dataset was preprocessed using Connectomist [12] for artifacts correction, registration between DWI and T1, and computation of local CSD model (spherical harmonics order = 6, regularization factor = 0.006) [14].
Global tractography method – A novel tractography method was developed using a spin-glass model [3,4,5,6] with a non generative approach to seed spins. We initialize a set of spins (8 per voxel) uniformly distributed in a propagation domain defined robustly using a mask computed from T1-weighted data [15]. Initial spin directions are computed using a Gibb's sampler randomly choosing one of the most likely directions provided by the underlying diffusion model. The method was developed with a generic design allowing to use any diffusion model (DTI, Q-ball, CSD, SHORE...). As in [6], the optimization algorithm is composed of a connection energy imposing a low curvature between two connected spins and of a data attachment energy corresponding to -log(P(ps, ds)) where P is the probability stemming from the diffusion model at the spin position (ps) and direction (ds). The optimization process consists of randomly choosing between: connection, move, creation_and_connection or destruction (especially for isolated spins within the white matter). The modification type is randomly chosen using a predefined set of probabilities pconnect(0.45)/pmove(0.3)/pcreate_and_connect(0.15)/pdestroy(0.10).
Embedded anatomical prior – Due to
the poor resolution of DWI data, the spins direction within
gyri cannot be accurately modeled by the underlying map of
diffusion models. Following the approach of [11], we constrain the spins direction during the optimization process by the knowledge of the cortical surface distance (d) and the normal direction
at the closest vertex of the cortex (dn): ds' = α * ds + ( 1 – α ) * dn. α is the
regularization factor and ds corresponds to the direction
driven by a Gibb's sampler built from the probabilities of the
underlying angular profile given by the chosen local model limited to the directions contained in a cone aligned with the former ds and of aperture angle
(20°).
To get a direction equal to ds when
far from the cortex and equal to dn when close to it, we used a sigmoid function: $$ψ(d) = 2 * exp( -
λ d ) / ( 1 + exp( -λ d )$$
Results and Discussion
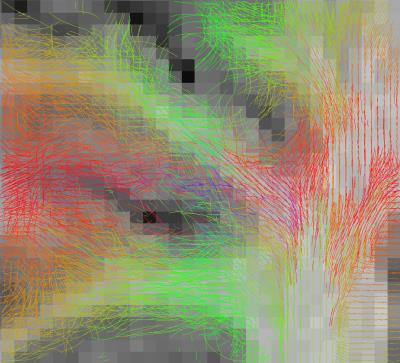
Figure 1 and 2 depict the initial spins configuration with and without the anatomical constraint in a gyrus region, showing the modification of the spins directions when adding the constraint. Figure 3 and 4 displays the results of the tracking using the global approach with and without the anatomical constraint. Whereas the global approach creates some rectilinear fibers mainly connecting the end of the gyrus but missing the sharp turns described in [16], the anatomically constrained one partially succeeds in reconstructing both fibers with low curvature far from the cortex and taking sharp turns when entering the gyrus or getting closer to the pial surface but still needs future improvements. Unlike [11] where the constraint is only performed during the initial seeding procedure, our anatomical constraint is active during the whole optimization process and at low computational cost. In addition, it was embedded in a global tractography approach, thus avoiding the pitfalls of “blind” deterministic and probabilistic streamlining methods.
Conclusion
In this work, we proposed a novel global tractography method allowing to monitor the fibers curvature depending on their location in order to create anatomically plausible connections and allowing to depict sharps turn when entering gyri and connecting to the cortex. In the future, we will add similar anatomical constraints in order to better handle the behavior of afferent and efferent connections to deep structures, such as the deep nuclei.Acknowledgements
This work was partially funded by the SAMENTA program of the French National Research Agency (ANR) - project HYPSY ANR-13-SAMA-0013
References
1: Behrens et al, 2007. Neuroimage, 34(1), 144-155.
2: Perrin et al, 2008. Journal of Biomedical Imaging, 2008, 4.
3: Fillard et al, 2009. In International conference on medical image computing and computer-assisted intervention (pp. 927-934). Springer Berlin Heidelberg.
4: Mangin et al, 2013. Toward global tractography. NeuroImage, 80, 290-296.
5: Kreher et al, 2008. Magnetic Resonance in Medicine, 60(4), 953-963.
6: Reisert et al, 2011. Neuroimage, 54(2), 955-962.
7: Daducci et al, 2016. Frontiers in Neuroscience, 10, 247.
8: Girard et al, 2015. In International Conference on Information Processing in Medical Imaging (pp. 675-686). Springer International Publishing.
9: Girard et al, 2014. Neuroimage, 98, 266-278.
10: Glasser et al, 2013 NeuroImage.
11: St-Onge et al, 2015. ISMRM conference 2015.
12: Assaf et al, 2013. Neuroimage 80:273-82;
13: Fischl B, 2012. Neuroimage 62(2):774-81;
14: Descoteaux et al, 2009. IEEE transactions on medical imaging, 28(2), 269-286.
15: Guevara et al, 2012. Neuroimage, 61(4), 1083-1099.
16: McNab et al, 2013. Neuroimage.
Figures


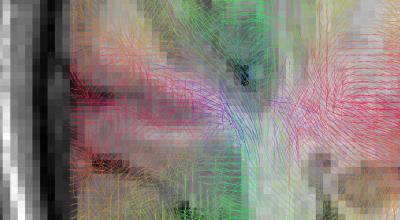
Reconstructed fibers without anatomical priors: tracts enter the gyrus in a straight direction and keep following parallel directions to the cortex until the end of it