0056
White matter changes and correlations with cognitive functions in semi-acute mild traumatic brain injury (mTBI): A hybrid diffusion imaging study1Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, United States, 2Department of Psychological and Brain Sciences, Dartmouth College, Lebanon, NH, United States, 3Department of Biostatistics, Indiana University, Indianapolis, IN, United States, 4Department of Psychiatry, Dartmouth Hitchcock Medical Center and New Hampshire Hospital, Lebanon, NH, United States, 5Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, United States
Synopsis
In the present study, we used multi-shell Hybrid Diffusion Imaging (HYDI) to study changes in white matter after mild traumatic brain injury (mTBI). From HYDI data, an array of diffusion metrics was computed including diffusion tensor imaging (DTI), neurite orientation distribution and density (NODDI), and return-to-origin (P0) of the q-space analysis. We study between group differences in diffusion metrics and within-group correlations with outcomes of cognitive functions. In addition, we tested the group effects (i.e., interaction or moderation) on the correlations between diffusion metrics and cognitive functions.
Purpose
Traumatic brain injury (mTBI) is a significant public health problem with about 1.6 million injuries occurring each year in the US, the majority (~80%) of which are categorized as “mild”. Despite the name, mild, and often normal findings in conventional neuroimaging exams, individuals with mTBI may experience disabling symptoms including headache, difficulty thinking, memory problems, attention deficits, mood swings and frustration. These symptoms could have long-term consequences in 10-20% of the people with mTBI1,2. In this study, we use a versatile hybrid diffusion imaging (HYDI) technique to study the white matter changes in the semi-acute stage of mTBI. In particular, we investigate an array of diffusion metrics computed from the HYDI data using diffusion tensor imaging (DTI), neurite orientation distribution and density (NODDI)3, and return-to-origin (P0) of the q-space analysis4,5. We study between group differences in diffusion metrics and within-group correlations with cognitive functions including: processing speed and cognitive flexibility, sustain attention, and memory. In addition, we test the group effects (i.e., interaction or moderation) on the correlations between diffusion metrics and cognitive functions.
Method
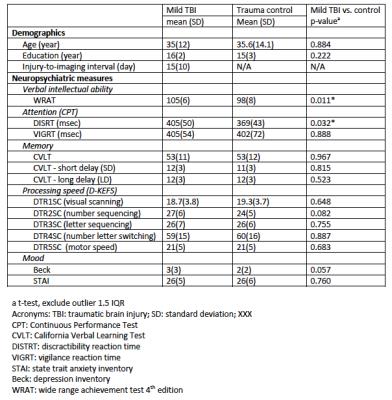
Participants: A total of 42 patients were recruited from the Emergency Department of the Dartmouth-Hitchcock Medical Center, Level 1 trauma center. Among them, there were 19 patients (11 females, 8 males) with mild traumatic brain injury and 23 trauma-controlled patients (11 females, 12 males). The clinical assessments were performed within a month of injury. The assessments included a full battery of neuropsychological measures similar to our previous publications6-8. The subject demographics and neuropsychological measures are listed in Figure 1. MRI: Participants received HYDI scans in a Philips 3T Achieve TX scanner with 8-channel head coil and SENSE parallel imaging. The diffusion-weighting (DW) pulse sequence was a SS-SE-EPI sequence. Other MR parameters were: TE/TR=114.24/3590ms, δ/Δ=46/58.4ms, voxel size=2x2x3mm2, 40 slices with slice thickness=3mm, parallel-imaging SENSE factor=2. The HYDI diffusion encoding scheme consisted 1 b0 and 5 b-value shells (250, 1000, 2250, 4000, and 6250 s/mm2) with total 142 DW directions (6, 21, 24, 30, and 61 in each shell)4. Image processing: The diffusion-weighted images were de-noised using local PCA denoiser9 followed by motion, eddy, and fieldmap correction using FSL EDDY10. The diffusion metrics (of DTI, NODDI, and P0) were computed similar to 11 followed by non-linear transformed to standard MNI space using ANTs registration12. Data Analysis: A common whole-brain while-matter skeleton map was extracted using FSL Tract-based spatial statistics (TBSS)13. TBSS was also used for voxelwise statistical analysis within the white-matter skeleton to study group differences in diffusion metrics, within-group correlation to neuropsychological measures, and group differences/interactions in correlations. The tests were adjusted for sex, age, IQ, and multiple comparisons.Result and Discussion
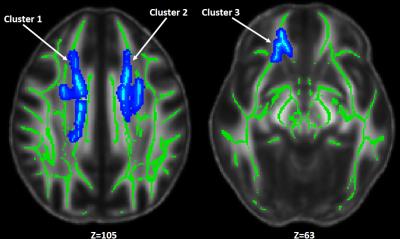
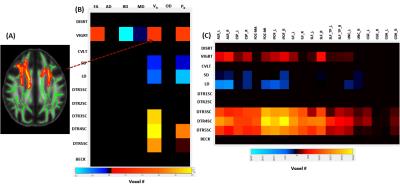
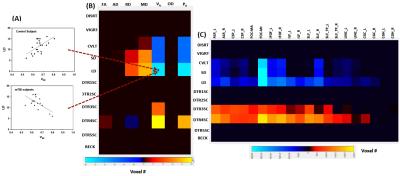
DTI metrics included fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD); and NODDI metrics included axonal density index (Vic) and orientation distribution index (ODI). Among these diffusion metrics, only Vic had significant differences between groups suggesting decreased axonal density in the mTBI subjects (Figure 2). Three significant clusters were observed covering fiber tracts of the forceps minor, inferior frontal-occipital, and cingulum-cingulate gyrus. Figure 3 shows results of correlations between diffusion metrics and neuropsychological measures. Compared to DTI metrics, Vic (axonal density) and P0 (tissue restriction) are more sensitive to neuropsychological measures including attention, memory, and processing speed (Figure 3B). Vic had a widespread white matter distribution with positive correlation with processing speed, while had minor distributions in white matter with positive correlation with attention and negative correlation with memory (Figure 3C). Figure 4 shows results of significant group interactions where diffusion metrics in mTBI correlated with neuropsychological measures in different strength or direction compared to the control group. For example, in Figure 4A, Vic had positive correlation with LD (memory) in the control group, but had negative correlation in the mTBI group. Shown in the summary matrix in Figure 4B, unlike memory and processing speed, the attention measures did not show any group difference in correlation with any diffusion metric. In addition, similar to Figure 3, Vic and P0 were more sensitive to group interactions with more neuropsychological measures and in more white-matter voxels. Vic had approximately equal distributions across the white matter tracts that showed group difference in the correlation with memory and processing speed (Figure 4C).
Conclusion
The axonal density inferred by Vic demonstrates high sensitivities to semi-acute mTBI and correlations of the neuropsychological outcomes. While there is no significant difference of the neuropsychological measures between the groups (except DISRT), there are overwhelming group differences in the correlations between the diffusion metrics and neuropsychological outcomes.
Acknowledgements
The work is supported by NIH R21 NS075791 and IUPUI-ITDP grantsReferences
1. McAllister, T.W., Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry, 2008. 7(1): p. 3-10.
2. Zemek, R.L., K.J. Farion, M. Sampson, and C. McGahern, Prognosticators of persistent symptoms following pediatric concussion: a systematic review. JAMA Pediatr, 2013. 167(3): p. 259-65.
3. Zhang, H., T. Schneider, C.A. Wheeler-Kingshott, and D.C. Alexander, NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage, 2012. 61(4): p. 1000-16.
4. Wu, Y.C. and A.L. Alexander, Hybrid diffusion imaging. Neuroimage, 2007. 36(3): p. 617-29.
5. Ozarslan, E., C.G. Koay, T.M. Shepherd, M.E. Komlosh, M.O. Irfanoglu, C. Pierpaoli, and P.J. Basser, Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure. Neuroimage, 2013. 78: p. 16-32.
6. McAllister, T.W., L.A. Flashman, B.C. McDonald, and A.J. Saykin, Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J Neurotrauma, 2006. 23(10): p. 1450-67.
7. McAllister, T.W., M.B. Sparling, L.A. Flashman, S.J. Guerin, A.C. Mamourian, and A.J. Saykin, Differential working memory load effects after mild traumatic brain injury. Neuroimage, 2001. 14(5): p. 1004-12.
8. McAllister, T.W., A.J. Saykin, L.A. Flashman, M.B. Sparling, S.C. Johnson, S.J. Guerin, A.C. Mamourian, J.B. Weaver, and N. Yanofsky, Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology, 1999. 53(6): p. 1300-8.
9. Manjon, J.V., P. Coupe, L. Concha, A. Buades, D.L. Collins, and M. Robles, Diffusion weighted image denoising using overcomplete local PCA. PLoS One, 2013. 8(9): p. e73021.
10. Yamada, H., O. Abe, T. Shizukuishi, J. Kikuta, T. Shinozaki, K. Dezawa, A. Nagano, M. Matsuda, H. Haradome, and Y. Imamura, Efficacy of distortion correction on diffusion imaging: comparison of FSL eddy and eddy_correct using 30 and 60 directions diffusion encoding. PLoS One, 2014. 9(11): p. e112411.
11. Kodiweera, C., A.L. Alexander, J. Harezlak, T.W. McAllister, and Y.C. Wu, Age effects and sex differences in human brain white matter of young to middle-aged adults: A DTI, NODDI, and q-space study. Neuroimage, 2016. 128: p. 180-92.
12. Avants, B.B., N.J. Tustison, G. Song, P.A. Cook, A. Klein, and J.C. Gee, A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 2011. 54(3): p. 2033-44.
13. Smith, S.M., M. Jenkinson, H. Johansen-Berg, D. Rueckert, T.E. Nichols, C.E. Mackay, K.E. Watkins, O. Ciccarelli, M.Z. Cader, P.M. Matthews, and T.E. Behrens, Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 2006. 31(4): p. 1487-505.
Figures