0050
Acute white matter abnormalities in sport-related concussion: A DTI study1Concussion Assessment, Research and Education (CARE) Consortium, Indianapolis, IN, United States, 2Department of Radiology and Imaging Sciences, Indiana University, Indianapolis, IN, United States, 3Department of Biostatistics, Indiana University, 4The Medical College of Wisconsin, 5Department of Psychiatry, Indiana University
Synopsis
In the present study, we use diffusion tensor imaging (DTI) to detect acute white matter alterations in football players after sport-related concussion. DTI scans were performed on 30 male football players who had acute concussion (24-48 hours post-injury). Another 28 matched contact-sport players were recruited as controls. Mean diffusivity (MD) increased significantly in concussive group compared to the contact-control group. Long fibers including corpus callosum, corona radiata, and longitudinal fasciculus were more vulnerable than the rest of the brain white matter. Within the concussed group, axial diffusivity (AD) demonstrated positive correlation with symptom severity indicating potential axonal changes/damage.
Purpose
Sport-related
concussion, albeit the mildest form of the brain injury, is a fundamental
concern and major public health issue. While most patients recover, a substantial
minority (10-20%) has disabling symptoms that could have long-term consequences1,2. In particular,
the nature of repetitive head impacts in contact sports may lead to chronic traumatic
encephalopathy3,4. Clinical
symptoms of concussion are generally subtle and hard to detect with common
assessment tools, more so in the acute stage. Thus, objective and sensitive neuroimaging
biomarkers to characterize concussion and monitor its recovery are of primary
research interest. Diffusion tensor imaging (DTI) was shown to effectively
differentiate between patients with mild traumatic brain injury (mTBI) and
controls5-7. In this
study, we use DTI to detect acute white matter alterations in football players
after sport-related concussion. In
addition, to assess its clinical correlation, we test significance of interaction
between DTI metrics and clinical outcome measure.Method
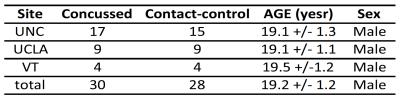
Participants: The present multi-site study was conducted under the umbrella of Concussion Assessment, Research and Education (CARE) consortium. Football players were recruited from three advanced research core (ARC) sites: University of North Carolina, University of California Los Angeles, and Virginia Tech. A comprehensive battery of clinical and neuropsychological assessments was acquired from each participant at baseline (pre season) and at acute concussion, i.e., 24-48 hours post-injury. DTI scans were performed within 48 hours post-injury. In addition, contact-sport players with matched sport type, number of prior concussion, and Wechsler Test of Adult Reading (WTAR) score were also recruited and received the same assessments and DTI scans. In this study, data from 30 concussed football players and 28 contact-sport controls were included in the analyses. DTI scans and parameters: DTI scans were performed in Siemens TimTrio and Prisma scanners across three sites and a 12CH receiver-only head coil with a SS-SE-EPI sequence. The diffusion encoding scheme consisted 30 directions at b-value= 1000s/mm2 and 4 b0 volumes (b-value= 0s/mm2). One of the b0 volumes was acquired with a reversed phase-encoding direction. Other parameters included TE/TR= 98/7900ms, FOV= 243mm, matrix size= 90x90, whole brain coverage of 60 slices with slice thickness of 2.7mm, and isotropic voxel at 2.7mm. Image processing: The diffusion-weighted images were de-noised using local PCA denoiser8 followed by motion, eddy, and fieldmap correction using FSL EDDY9. DTI metrics computed by FSL DITFIT were non-linearly transformed to standard MNI space using ANTs registration10. Data Analysis: A common whole-brain while-matter skeleton map was extracted using FSL Tract-based spatial statistics (TBSS)11. TBSS was also used for voxelwise statistical analysis within the white-matter skeleton testing for group differences in diffusion metrics, within-group correlation between diffusion metrics and symptom severity score, and between group interactions in correlation. The tests were adjusted for sites, scanners, and baseline scores.Results
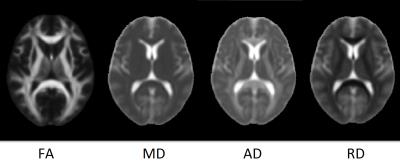
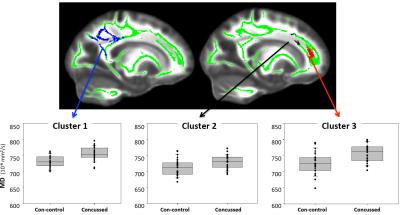
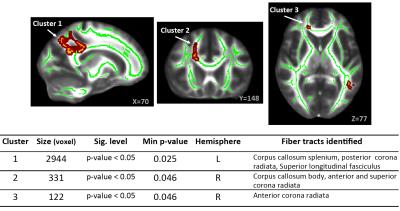
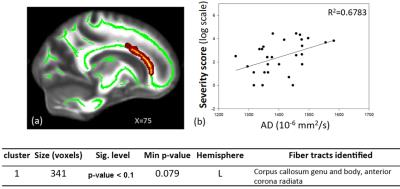
Summary of the sample size and age across sites is listed in Figure 1. Averaged DTI metrics including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RA) are shown in Figure 2. Among these DTI metrics, only MD demonstrated significant increase in the concussed group with corrected p-value < 0.05 (Figure 3). The amount of increase was between 5 and 7%. Three clusters were identified and located majorly in the corpus callosum (body and splenium), corona radiata (anterior, posterior, and superior), and superior longitudinal fasciculus (Figure 4). AD had marginally significant positive correlation with symptom severity (R2=0.68, corrected p-value < 0.10) in the concussed group (Figure 5). The significant cluster located in the corpus callosum (genu and body) and anterior corona radiata (Figure 5).
Discussion and conclusion
In this carefully designed study on football players, DTI demonstrates significant sensitivity to white matter alterations in the acute stage of concussion. Consistent with previous publications in acute and semi-acute mild traumatic brain injury (mTBI)5-7, long fibers including the corpus callosum, corona radiata, and longitudinal fasciculus are more vulnerable to biomechanical insult. Factional anisotropy (FA) is widely used in acute to semi-acute mTBI, but has inconsistent results reported with positive, negative and no changes in previous publications6. Consistent with the recent publications in acute MTBI (<72 hours)12-14, higher MD is found in the concussed group. Positive correlation of AD and symptom severity may indicate possible axonal changes/damage in line with prior animal studies15. However, such indication may be confounded with other factors such as inflammation16 or crossing fibers17. Therefore, while DTI is consistently showing high sensitivity to subtle white matter changes in mTBI, due to its simplified model, improved diffusion modeling with more specific biologic connection may help to elucidate the underlying mechanism of sport-related concussion and MTBI.
Acknowledgements
The National Collegiate Athletic Association (NCAA) and the Department of Defense (DoD)References
References:
1. McAllister, T.W., Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry, 2008. 7(1): p. 3-10.
2. Zemek, R.L., K.J. Farion, M. Sampson, and C. McGahern, Prognosticators of persistent symptoms following pediatric concussion: a systematic review. JAMA Pediatr, 2013. 167(3): p. 259-65.
3. McKee, A.C., R.C. Cantu, C.J. Nowinski, E.T. Hedley-Whyte, B.E. Gavett, A.E. Budson, V.E. Santini, H.S. Lee, C.A. Kubilus, and R.A. Stern, Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol, 2009. 68(7): p. 709-35.
4. McKee, A.C., R.A. Stern, C.J. Nowinski, T.D. Stein, V.E. Alvarez, D.H. Daneshvar, H.S. Lee, S.M. Wojtowicz, G. Hall, C.M. Baugh, D.O. Riley, C.A. Kubilus, K.A. Cormier, M.A. Jacobs, B.R. Martin, C.R. Abraham, T. Ikezu, R.R. Reichard, B.L. Wolozin, A.E. Budson, L.E. Goldstein, N.W. Kowall, and R.C. Cantu, The spectrum of disease in chronic traumatic encephalopathy. Brain, 2013. 136(Pt 1): p. 43-64.
5. Eierud, C., R.C. Craddock, S. Fletcher, M. Aulakh, B. King-Casas, D. Kuehl, and S.M. LaConte, Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin, 2014. 4: p. 283-94.
6. Dodd, A.B., K. Epstein, J.M. Ling, and A.R. Mayer, Diffusion tensor imaging findings in semi-acute mild traumatic brain injury. J Neurotrauma, 2014. 31(14): p. 1235-48.
7. Bigler, E.D., Neuroimaging biomarkers in mild traumatic brain injury (mTBI). Neuropsychol Rev, 2013. 23(3): p. 169-209.
8. Manjon, J.V., P. Coupe, L. Concha, A. Buades, D.L. Collins, and M. Robles, Diffusion weighted image denoising using overcomplete local PCA. PLoS One, 2013. 8(9): p. e73021.
9. Yamada, H., O. Abe, T. Shizukuishi, J. Kikuta, T. Shinozaki, K. Dezawa, A. Nagano, M. Matsuda, H. Haradome, and Y. Imamura, Efficacy of distortion correction on diffusion imaging: comparison of FSL eddy and eddy_correct using 30 and 60 directions diffusion encoding. PLoS One, 2014. 9(11): p. e112411.
10. Avants, B.B., N.J. Tustison, G. Song, P.A. Cook, A. Klein, and J.C. Gee, A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 2011. 54(3): p. 2033-44.
11. Smith, S.M., M. Jenkinson, H. Johansen-Berg, D. Rueckert, T.E. Nichols, C.E. Mackay, K.E. Watkins, O. Ciccarelli, M.Z. Cader, P.M. Matthews, and T.E. Behrens, Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 2006. 31(4): p. 1487-505.
12. Toth, A., N. Kovacs, G. Perlaki, G. Orsi, M. Aradi, H. Komaromy, E. Ezer, P. Bukovics, O. Farkas, J. Janszky, T. Doczi, A. Buki, and A. Schwarcz, Multi-modal magnetic resonance imaging in the acute and sub-acute phase of mild traumatic brain injury: can we see the difference? J Neurotrauma, 2013. 30(1): p. 2-10.
13. Veeramuthu, V., V. Narayanan, T.L. Kuo, L. Delano-Wood, K. Chinna, M.W. Bondi, V. Waran, D. Ganesan, and N. Ramli, Diffusion Tensor Imaging Parameters in Mild Traumatic Brain Injury and Its Correlation with Early Neuropsychological Impairment: A Longitudinal Study. J Neurotrauma, 2015. 32(19): p. 1497-509.
14. Narayana, P.A., X. Yu, K.M. Hasan, E.A. Wilde, H.S. Levin, J.V. Hunter, E.R. Miller, V.K. Patel, C.S. Robertson, and J.J. McCarthy, Multi-modal MRI of mild traumatic brain injury. Neuroimage Clin, 2015. 7: p. 87-97.
15. Budde, M.D., M. Xie, A.H. Cross, and S.K. Song, Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci, 2009. 29(9): p. 2805-13.
16. Wang, Y., P. Sun, Q. Wang, K. Trinkaus, R.E. Schmidt, R.T. Naismith, A.H. Cross, and S.K. Song, Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain, 2015.
17. Wheeler-Kingshott, C.A. and M. Cercignani, About "axial" and "radial" diffusivities. Magn Reson Med, 2009. 61(5): p. 1255-60.
Figures