0046
Non-contrast free breathing and motion corrected 3D whole heart quantitative magnetization transfer imaging for assessment of myocardial fibrosis1Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
We have developed a contrast-free free-breathing motion corrected (100% scan efficiency) 3D whole heart imaging technique for measurement of myocardial magnetization transfer ratio (MTR) maps. The sequence is based on the interleaved acquisition of MT weighted and non-MT weighted datasets and beat-to-beat rigid motion correction using 2D image navigators. Initial results in 4 healthy volunteers have shown good image quality, enabling the visualisation of the coronary arteries and MTR maps of healthy myocardium (MTR=41±7.2%). This approach promises higher sensitivity for measuring changes in macromolecule content associated with myocardial fibrosis than previous studies, justifying further investigation in a patient cohort.
Purpose
Late Gadolinium Enhancement (LGE) MRI is the gold standard technique for non-invasive assessment of focal myocardial fibrosis. However LGE MRI is not quantitative, relies on the differential uptake of contrast agent in healthy and diseased myocardium and therefore cannot assess diffuse fibrosis. Native and post-contrast myocardial T1-mapping have been proposed to address this limitation and enable the measurement of the extracellular volume (ECV), which is a good imaging biomarker for collagen deposition and fibrosis. However, ECV quantification requires the administration of an exogenous contrast, which is a contraindication in patients with renal dysfunction. In addition, T1 mapping requires the acquisition of multiple (typically 9-11) T1 weighted images, which leads to very long scan times if used for whole heart coverage. Alternatively, magnetization-transfer (MT) contrast has proven to have high sensitivity and specificity for collagen content in thrombus [1] and human cartilage [2]. MT-ratio has also shown agreement with LGE in patients with acute myocardial infarction [3]. Furthermore, MT-ratio using a 2D cine technique was compared successfully to LGE [4]. In this work we developed and evaluated a non-contrast free breathing and motion corrected 3D whole heart quantitative magnetization transfer imaging approach for assessment of myocardial fibrosis.Methods
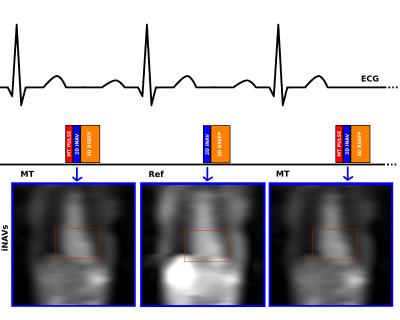
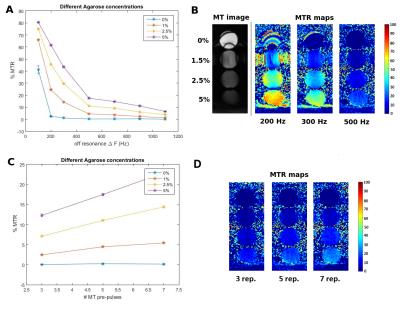
Proposed sequence: A free-breathing interleaved acquisition of two segmented 3D balanced SSFP datasets were implemented (Figure 1). An off-resonance MT preparation was applied at every other heartbeat to generate one MT-weighted dataset (MT) and one reference dataset (Ref), respectively. 2D image navigators (acquired during the ramp-up pulses of the 3D bSSFP readout) were acquired at each heartbeat and used for 2D translational beat-to-beat motion compensation and ensure 100% scan efficacy[5]. 3D Pixel-by-pixel MT-ratio (MTR) maps were calculated as $$MTR=100*\frac{(Ref-MT)}{Ref}$$, following image registration using a non-rigid fast free-form deformation algorithm (NiftyReg) [6]. Off-resonance frequencies ΔF between 200 and 400 Hz and pulse repetitions >7 were found optimal to provide maximum MTR in an agar-based phantom with three different amounts of macromolecular tissue content (Figure 2).
Four healthy volunteers and one patient were scanned with the new prototype 3D MTR sequence on a 1.5T MR scanner (Magnetom Aera, Siemens Healthcare, Erlangen, Germany): FoV=300x300 mm2, resolution=1.4x1.4x2 mm3, TR/TE=3.38/1.69 ms, flip angle 70°, bandwidth=1415 Hz/px. The following MT pre-pulse parameters were used: Gaussian pulse, off-resonance frequency= 200 Hz, 10 pulse repetitions, flip angle=720°, pulse duration=20.48 ms and bandwidth-time product=1.92. Average scan time was 12 minutes without image acceleration and 100% scan efficiency.
Results
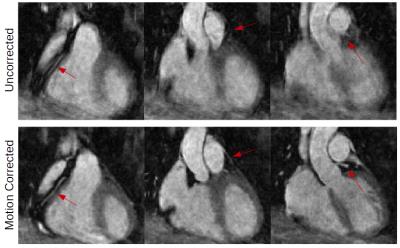
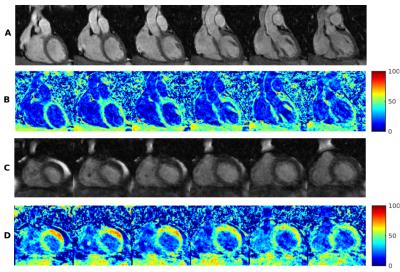
Motion correction on MT-weighted datasets was successful in recovering features such as the coronary arteries, myocardial boundaries and in increasing image quality in all volunteers, as can be seen in a representative example in Figure 3. The average MTR value in volunteers was [41±7.2] %. In the patient dataset, a region in the left ventricle with values above 80% was observed (Figure 4d), which could indicate the presence of fibrotic tissue. Figure 4[a-d] shows a comparison of coronal slices from the patient dataset with focally increased MTR values (~80%) and coronal slices from one of the volunteers with homogenously distributed MTR values around 40%.Discussion
We successfully developed a 3D whole-heart contrast-free imaging sequence for MTR mapping, which was evaluated in four volunteers and one patient. The novel sequence provides 1) beat-to-beat translational motion correction with 100% scan efficiency and allows to acquire data during free-breathing, 2) high MT contrast for visualization of both myocardium and coronary arteries and 3) the sequence was found to be reproducible and does not require any external contrast. MTR values were higher than found by Weber et. al. (MTR=33±3 %) who employed a bSSFP sequence to generate MT contrast [3]. This is possibly due to the stronger saturation of the macromolecular pool with the optimized off-resonant MT preparation. This is encouraging because it means a potential increase in the sensitivity to changes in the macromolecular content for example in diseased myocardium. The results of this initial study are promising but a larger number of patients is needed to validate the MTR contrast for the detection of focal and diffuse fibrosis. The precision of the MTR values obtained in the myocardium of healthy subjects might also be improved by a non-rigid motion correction framework [7].Conclusion
We successfully demonstrated the use of a novel motion corrected 3D whole-heart contrast-free interleaved MT sequence for the measurement of MT ratio in healthy subjects and patients. Promising results in the first patient warrant further investigation in a larger patient cohort.Acknowledgements
This work was supported by the EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1), Siemens Healthcare GmbH and by EPSRC grants EP/P001009/1 and EP/P007619/1.References
[1] Phinikaridou, A., et al. "In vivo magnetization transfer and diffusion-weighted magnetic resonance imaging detects thrombus composition in a mouse model of deep vein thrombosis." Circulation: Cardiovascular Imaging 6.3 (2013): 433-440.
[2] Welsch, Goetz H., et al. "Magnetization transfer contrast and T2 mapping in the evaluation of cartilage repair tissue with 3T MRI." Journal of magnetic resonance imaging 28.4 (2008): 979-986.
[3] Weber, O.M., et al. "Assessment of magnetization transfer effects in myocardial tissue using balanced steady state free precession (bSSFP) cine MRI." Magnetic resonance in medicine 62.3 (2009): 699-705.
[4] Stromp, Tori A., et al. "Gadolinium free cardiovascular magnetic resonance with 2-point Cine balanced steady state free precession." Journal of Cardiovascular Magnetic Resonance 17.1 (2015): 1.
[5] Henningsson, Markus, et al. "Whole-heart coronary MR angiography with 2D self-navigated image reconstruction." Magnetic resonance in medicine 67.2 (2012): 437-445.
[6] Ourselin, S., et al. "Reconstructing a 3D structure from serial histological sections." Image and vision computing 19.1 (2001): 25-31.
[7] Cruz, Gastao, et al. "Highly efficient nonrigid motion-corrected 3D whole-heart coronary vessel wall imaging." Magnetic Resonance in Medicine (2016).
Figures