0042
Multiparametric Characterization of Myocardial Tissue by Contrast-Enhanced Cardiac Magnetic Resonance Imaging in Subjects with Prediabetes, Diabetes and Controls from a Western General Population – Results of the KORA-MRI-Study1Radiology, University Hospital Tuebingen, Tuebingen, Germany, 2Clinical Radiology, Ludwig-Maximilians-University Munich, Germany, 3Biometry and Epidemiology, German Diabetes Center Duesseldorf, Germany, 4Diagnostic and Interventional Radiology, University Hospital Heidelberg, Germany, 5Epidemiology II, Helmholtz Zentrum Munich, German Research Center for Environmental Health, Neuherberg, Germany, 6Cardiovascular Prevention, Ludwig-Maximilians University Munich, Munich, Germany, 7German Center for Cardiovascular Disease Research (DZHK e.V.) Munich, Munich, Germany, 8Diagnostic and Interventional Radiology, University Hospital Tuebingen, Tuebingen, Germany, 9Cardiology, Charité, Experimental and Clinical Research Center and HELIOS-Clinics Berlin-Buc, Germany, 10German Center for Cardiovascular Disease Research (DZHK e. V.) Partnersite Berlin, Germany
Synopsis
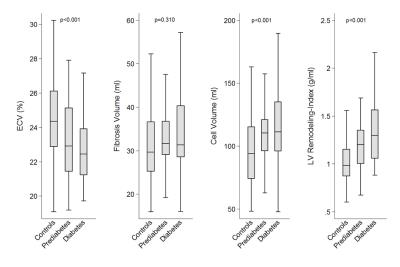
Cardiac magnetic resonance imaging (CMR) allows for detailed characterization of the myocardium, which may be beneficial in assessing cardiomyopathy in the setting of hyperglycemic states. We performed a comprehensive CMR protocol in subjects with prediabetes, diabetes and controls and preserved left ventricular (LV) ejection fraction (EF) in a western population-based sample. Subjects with prediabetes and diabetes had an increased LV-remodeling-index as well as higher estimates of cell volume compared with controls, while extracellular volume, as a parameter of diffuse myocardial fibrosis (MF), was decreased. This may highlight the role for hypertrophy in the pathogenesis of diabetic cardiomyopathy in this western population.
Purpose
Diabetic cardiomyopathy represents a major risk in the natural history of diabetes1,2. Besides diabetes, there is also a substantial proportion of individuals with impaired glucose metabolism, classified as prediabetes, incurring an increased risk for progressing to diabetes type 2 and cardiovascular events3,4. Even in patients with preserved left ventricular ejection fraction (LVEF), cardiac magnetic resonance imaging (CMR) allows for the detection of early changes in the myocardium by the assessment of focal and diffuse myocardial fibrosis (MF) as well as changes in cellular volume (CV)5-7. However, detailed information on myocardial tissue changes remains largely unclear, particularly in subjects with prediabetes. The aim of this study was to detect early changes in the myocardium in subjects with prediabetes, diabetes, and healthy controls with preserved LVEF by using CMR in a sample from the general population.Methods
Subjects without history of cardiovascular disease and preserved LVEF but established diabetes, prediabetes and normal controls from a population-based cohort (Cooperative Health Research in the Region of Augsburg (KORA)) underwent contrast-enhanced whole-body 3 Tesla CMR. Obtained parameters included left ventricular (LV) function and morphology like the LV-remodeling-index (=LV mass/ LV end-diastolic volume), late gadolinium enhancement (LGE) for assessment of focal fibrosis as well as T1-mapping and derivation of extra-cellular volume fraction (ECV= (1 – hematocrit level) × (ΔR1myocardium / ΔR1 blood))) by the modified ECG-gated Look-Locker inversion recovery sequence (MOLLI) for diffuse fibrosis in a subset of patients. MF and CV (= ((1-ECV)*LVmass)/myocardial density) were calculated. The CMR protocol comprised cine-SSFP sequences in the short axis covering the left ventricle and a 4-chamber view followed by pre- and post-contrast T1 modified MOLLI and LGE sequences 10 minutes after administration of gadopentetate dimeglumine8. The MOLLI sequences were acquired on short axis at the mid-ventricular level using the following parameters: slice thickness 8 mm, spatial resolution: 1.5 x 1.5 mm, FOV: 323 x 380 mm using a 256 x 144 mm matrix TR: 250 – 400 TE: 1.1, TI: 100 – 3500 with a 35 degrees flip angle. LGE was acquired on Fast Low Angle Shot (FLASH) inversion recovery sequences with the following parameters: slice thickness 8mm, FOV 300x360mm, Matrix 256 x 140, TR 700-1000ms, TE 1.55 ms, FA 20-55°. For the image analysis of T1 mapping, the mean T1 values were obtained after tracing the inner and outer contour of the LV myocardium as well as of the blood-filled LV lumen in the mid-ventricular short axis T1 maps. Univariate and multivariate analyses were carried out in order to determine group differences.Results
Among 343 subjects (mean age: 56.1±9.2 years, 57% males), 47 subjects were classified as diabetic, 78 as prediabetes, and 218 controls (14%, 23%, and 64%; respectively). LVEF was preserved in all patients but LV-remodeling-index was significantly higher in participants with prediabetes and diabetes, independent of BMI, hypertension, age and sex. Neither T1 native relaxation times nor fibrosis volume (FV) were different among diabetes status groups. ECV was decreased in subjects with prediabetes and diabetes compared to healthy controls (23.1±2.4% and 22.8±3.0%, both p<0.007, respectively). In contrast, CV was significantly higher in subjects with prediabetes and in diabetics as compared to healthy controls (109.1±23.8 ml and 114.9±32.3 ml vs. 96.5±26.9 ml, both p<0.03, respectively).Discussion
Our results indicate that subjects with prediabetes and diabetes have increased LV-remodeling-indices as detected by CMR compared to healthy controls. This finding is in line with previous studies, demonstrating a strong association between insulin resistance, central obesity and LV-remodeling-indices, independent of metabolic risk factors9. In addition, we found higher CV levels in subjects with diabetes and prediabetes as compared to healthy controls. Previous research in human biopsy studies demonstrated an association of hypertrophy of myocardial cells and diabetes status, even in early stages of the diseases, suggesting a predominant role of cell hypertrophy in the pathology of diabetic cardiomyopathy6,7. In contrast to other studies10-13, ECV as a parameter of diffuse MF, was decreased in our population, while native T1 relaxation times or the myocardial partition coefficient λ was not different. These results might indicate that diffuse MF is less relevant in this well-treated western diabetic population with preserved LVEF, emphasizing the role of hypertrophy in pathogenesis of diabetic cardiomyopathy.Conclusion
Conclusion: Subjects with prediabetes and diabetes but preserved LVEF had higher LV-remodeling-indices and CV than controls, indicating a predominant role for hypertrophy in the pathogenesis of diabetic cardiomyopathy in this well-treated western population.Acknowledgements
No acknowledgement found.References
1. Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 2008;121:748-57.
2. Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 2004;25:543-67.
3. Danaei G, Lawes CM, Vander Hoorn S, Murray CJ, Ezzati M. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet 2006;368:1651-9.
4. Yoon YE, Kitagawa K, Kato S, et al. Prognostic significance of unrecognized myocardial infarction detected with MR imaging in patients with impaired fasting glucose compared with those with diabetes. Radiology 2012;262:807-15.
5. Lee SP, Lee W, Lee JM, et al. Assessment of diffuse myocardial fibrosis by using MR imaging in asymptomatic patients with aortic stenosis. Radiology 2015;274:359-69.
6. Nunoda S, Genda A, Sugihara N, Nakayama A, Mizuno S, Takeda R. Quantitative approach to the histopathology of the biopsied right ventricular myocardium in patients with diabetes mellitus. Heart Vessels 1985;1:43-7.
7. Fischer VW, Barner HB, Larose LS. Pathomorphologic aspects of muscular tissue in diabetes mellitus. Hum Pathol 1984;15:1127-36.
8. Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging 2007;26:1081-6.
9. Shah RV, Abbasi SA, Heydari B, et al. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;61:1698-706.
10. Schelbert EB, Messroghli DR. State of the Art: Clinical Applications of Cardiac T1 Mapping. Radiology 2016;278:658-76.
11. Schelbert EB, Piehler KM, Zareba KM, et al. Myocardial Fibrosis Quantified by Extracellular Volume Is Associated With Subsequent Hospitalization for Heart Failure, Death, or Both Across the Spectrum of Ejection Fraction and Heart Failure Stage. J Am Heart Assoc 2015;4.
12. Liu S, Han J, Nacif MS, et al. Diffuse myocardial fibrosis evaluation using cardiac magnetic resonance T1 mapping: sample size considerations for clinical trials. J Cardiovasc Magn Reson 2012;14:90.
13. Lamb HJ, Beyerbacht HP, de Roos A, et al. Left ventricular remodeling early after aortic valve replacement: differential effects on diastolic function in aortic valve stenosis and aortic regurgitation. J Am Coll Cardiol 2002;40:2182-8.