0039
Free-Breathing, Non-ECG-Gated, Continuous Myocardial T1 Mapping and ECV Quantification with Multitasking1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, UCLA, Los Angeles, CA, United States, 3Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA
Synopsis
Currently used T1 mapping techniques utilize both ECG gating and breath-holds/navigators with low imaging efficiency and/or respiratory motion artifacts. We removed the need for ECG gating and respiratory monitoring with Cardiac MR Multitasking, a continuous acquisition technique using a low-rank tensor (LRT) imaging framework. The aim of this work is to validate a free-breathing, non-ECG-gated native T1 mapping and ECV quantification method in healthy subjects against standard MOLLI T1 mapping.
Purpose
Myocardial T1 mapping is known to characterize both focal and diffuse myocardial fibrosis, important factors associated with adverse outcomes in cardiovascular disease.1 Additionally, extracellular volume (ECV) calculated from pre- and post-contrast T1 of myocardium and blood along with hematocrit has been shown to correlate with histologic measures of myocardial fibrosis.2 Currently used T1 mapping techniques utilize both ECG gating and breath-holds/navigators with low imaging efficiency and/or respiratory motion artifacts. We removed the need for ECG gating and respiratory monitoring with Cardiac MR Multitasking, a continuous acquisition technique using a low-rank tensor (LRT) imaging framework.3,4 The aim of this work is to validate a free-breathing, non-ECG-gated native T1 mapping and ECV quantification method in healthy subjects against standard MOLLI T1 mapping.Methods
The proposed continuous 2D single-slice acquisition utilizes a golden-angle radial Inversion Recovery FLASH scheme modified to also collect interleaved low-rank tensor auxiliary data used for temporal subspace estimation.3,4 Inversion recovery pulses are applied at set intervals to achieve T1 recovery. Sequence parameters include: scan time: 60 seconds, 2.5 s between IRs, 24 inversion recovery pulses, TE/TR: 1.6/3.6ms, flip angle: 5°, FoV: 270x270mm2, in-plane resolution: 1.7x1.7mm2, slice thickness: 8 mm.
First, real-time low-rank matrix images5 were reconstructed from the image data and auxiliary data for image-based cardiac and respiratory binning. Next, LRT reconstruction was performed using an explicit tensor subspace constraint estimated from the auxiliary data and a dictionary of Bloch equation T1 curves with 344 inversion time (TI) images (range: 3.6-2,476 ms, 7.2 ms temporal resolution), with 15 cardiac and 5 respiratory bins. Pixel-wise T1 maps were created by fitting to a FLASH-IR model at an end-expiration respiratory phase and a diastolic cardiac phase. For both methods, T1 was measured with an ROI in the septum. Repeatability was assessed as within-subject variance by the coefficient of variation (CV).
Ten healthy subjects (34.6±9.1, 50% female) were scanned at 3T (Siemens Verio) with the proposed method in a single mid-ventricular slice as well as a resolution-matched breath-hold, ECG-gated, MOLLI 5(3)3 with online motion correction.6 Both methods were repeated pre-contrast 3 times for native T1 mapping. In a subset of six subjects (37.0±10, 50% female), post-contrast T1 mapping was also performed 15 minutes following 0.2 mmol/kg of gadovist injection with first MOLLI then the proposed continuous method. ECV was calculated for both methods using the hematocrit measured directly prior to imaging.
Results
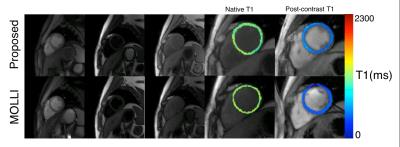
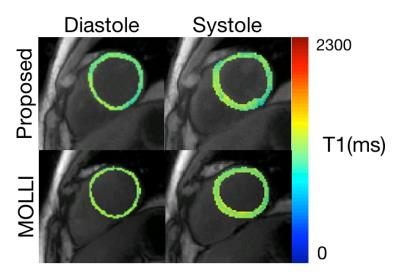
Figure 1 shows TI images as well as native and post-contrast T1 maps for the proposed method and MOLLI. Native T1 values for both methods are not significantly different (MOLLI: 1259.3±45.9, Proposed: 1246.7±24.6, p=0.53) and are within the published normal range at 3T. Both methods demonstrate low CVs within 3 repetitions, demonstrating good repeatability (MOLLI: 1.16%, Proposed: 2.48%). ECV values for both methods are not significantly different (MOLLI: 27.0±3.9, Proposed: 26.6±4.0, p=0.68). Figure 2 demonstrates the ability of the proposed method to quantify T1 in both diastole and systole from the same scan while MOLLI requires 2 separate breath-hold scans.Discussion and Conclusion
We demonstrate the ability of the proposed free-breathing, non-ECG-gated, T1 mapping technique to produce T1 and ECV values similar to MOLLI. Good repeatability is shown for non-contrast T1 mapping with the proposed technique. The proposed technique also enables T1 quantification throughout the cardiac cycle and at multiple respiratory phases. Our findings show promise for myocardial T1 mapping and ECV quantification free of ECG-gating and breath-holds or respiratory navigators.Acknowledgements
No acknowledgement found.References
1. Puntmann V, Peker E, Chandrashekhar Y, et al. T1 Mapping in Characterizing Myocardial Disease. Circ Res. 2016;119(2):277-299.
2. Shaw JL, Christodoulou AG, Sharif B, et al. Ungated, Free-breathing, Native T1 Mapping in Multiple Cardiac Phases in Under One Minute: A Proof of Concept. ISMRM 2016.
3. Christodoulou AG, Shaw JL, Sharif B, et al. A General Low-Rank Tensor Framework for High-Dimensional Cardiac Imaging: Application to Time-Resolved T1 Mapping. ISMRM 2016.
4. Christodoulou AG, Zhang H, Zhao B. High-Resolution Cardiovascular MRI by Integrating Parallel Imaging with Low-Rank and Sparse Modeling. IEEE Trans Biomed Eng. 2013;60(11):3083-3092.
5. Xue H, Greiser A, Zuehlsdorff S. Phase-Sensitive Inversion Recovery for Myocardial T1 Mapping with Motion Correction and Parametric Fitting. Magn Reson Med. 2013;69(5):1408-1420.
Figures