0038
Simultaneous Multi-Slice Imaging For Whole Heart Myocardial T1 Mapping In A Single Breath-Hold1Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3Computer Assisted Clinical Medicine, Heidelberg University, Mannheim, Germany, 4Department of Cardiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
Myocardial T1-mapping bears promise for evaluation of numerous cardiomyopathies, but requires multiple breath-hold scans with conventional techniques. In this study we explored the acceleration potential of multi-band imaging for myocardial SAPPHIRE T1-mapping with 3-slice coverage in a single breath-hold. Three linear methods for slice and in-plane unaliasing were evaluated. Phantom studies confirmed the accuracy of the proposed T1-mapping method, and in-vivo evaluation has shown reliable image quality with 3-fold acceleration at the cost of 1.3 to 1.7-fold increased in-vivo variability. Smaller loss in-vivo precision was achieved using regularized methods, for the trade-off against increased inter-slice leakage.
Background
Myocardial T1-mapping is increasingly used for detection and staging of diffuse cardiomyopathies1. Three slice coverage in short axis orientation is recommended for evaluation that mitigates the heterogeneity across left ventricle2. However, commonly used myocardial T1-mapping methods acquire only a single-slice per breath-hold, leading to patient discomfort and long scan times due to rest-periods. Simultaneous multi-slice (SMS) or multi-band (MB) imaging has been proposed for scan-time reduction by acquiring multiple slices simultaneously, where the only SNR reduction compared to single-slice imaging is due to coil geometry3. MB imaging became a popular tool for neurological applications4, but its use in cardiac applications has been limited5. In this study, we sought to investigate the potential of MB imaging in myocardial T1-mapping for comprehensive tissue characterization with 3-slice coverage in a single breath-hold.Methods
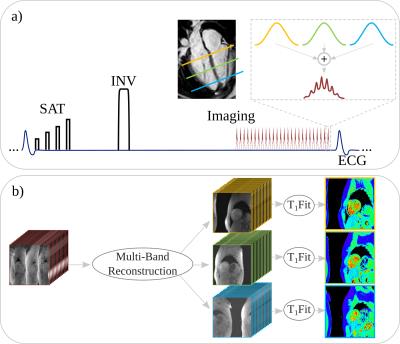
Figure 1 shows the diagram of the proposed technique: A hybrid saturation/inversion-recovery SAPPHIRE T1-mapping sequence was used6 with FLASH imaging readout. Slice-selective excitation was performed on three frequencies to enable multi-band acquisition. The RF phase in each band was cycled to shift the slices in the acquired image, as proposed in CAIPIRINHA3. B1+ peak amplitude was minimized by adding an optimized constant RF phase for each band. To enable slice unaliasing, a 1-second reference scan was used to acquire low-resolution images (6x6 mm2) of 3 slices, during free-breathing and without ECG-gating.
All imaging was performed at 3T (Siemens Magnetom Prisma). Phantom images were acquired to confirm accurate T1-mapping with the proposed multi-band approach and to compare the T1-mapping precision to single-band imaging. In-vivo imaging was performed in six healthy subjects (6 male, 32±12 y/o). Single breath-hold MB SAPPHIRE was compared to a conventional single-band (SB) SAPPHIRE acquisition in three breath-holds. Both sequences shared the following imaging parameters: Uniform in-plane undersampling = 2, FOV 320x320mm2, resolution=2x2mm2, slice thickness=10mm, partial-Fourier=6/8, TR/TE/FA=4/2ms/10°.
Three reconstruction techniques were compared : 1) Multi-slice unaliasing (Fig. 1b) was performed using slice-GRAPPA7, followed by in-plane GRAPPA8, whose kernels were calibrated from the low resolution reference scan, with (5,5) and (5,4) kernels, respectively. The final images were generated using a coil-sensitivity weighted combination of individual coil images. 2) A CG-SENSE9 reconstruction for both slice and in-plane unaliasing. Coil-sensitivity maps for each band and each coil were generated from the reference scan. 3) The reconstruction in (2) with additional Tikhonov regularization10.
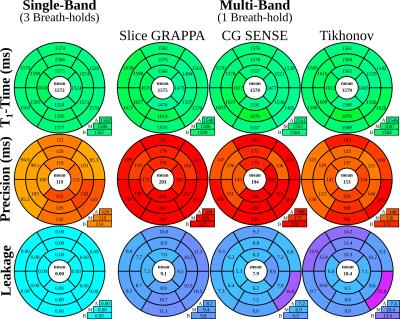
Subsequently, phase-sensitive fitting was performed on the final T1-weighted images to obtain T1-maps (Fig. 1b). Leakage maps were calculated using the respective MB reconstruction on spatially shifted SB images11. T1-time, precision and leakage in the myocardium were quantitatively analyzed according to the AHA 16-segment model.
Results
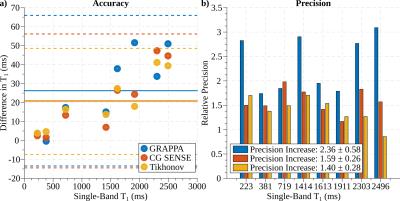
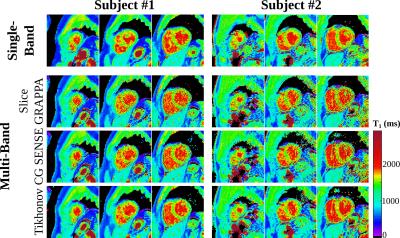
Good phantom accuracy was shown for all reconstruction methods, with similar normalized root mean square error of 1.5-2.0%. GRAPPA reconstruction results in higher loss of precision (2.4-fold) compared to CG-SENSE (1.6-fold). Tikhonov-regularized CG-SENSE showed the smallest increase in variability (1.4-fold). As apparent from the exemplary T1-maps, visually improved homogeneity is obtained using Tikhonov-regularized CG-SENSE, compared to the other reconstruction methods (Fig. 3). However, CG-SENSE shows increased slice leakage, as depicted in subject #2, although outside the myocardial area. Quantitative analysis of the in-vivo T1-times, shows good agreement of all reconstruction methods with the conventional single-band sequence (Fig 4.). The differences in in-vivo precision between single- and multi-band imaging are less pronounced (GRAPPA:1.7-fold, CG-SENSE:1.6-fold, Tikhonov:1.3-fold increase) compared with phantom. Tikhonov-regularized CG-SENSE shows the highest inter-slice leakage, with particular elevation in the basal slice.Discussion
The proposed technique enables acquisition of myocardial T1-maps with coverage of the 16 AHA-segments in a single breath-hold. More than 3-fold savings in acquisition time is achieved, at a loss of T1-precision of 1.3 to 1.7-fold. The position of the heart at end-expiration is known to be subject to major variations even in healthy volunteers. Hence, T1-map acquisition of three short-axis slices in separate breath-holds provides potentially non-equidistant coverage with bias towards basal or apical T1-times. As all slices are acquired simultaneously in MB T1-mapping, equidistant and uniform coverage of the left-ventricle is ensured in a short-axis stack scan with the proposed technique.
Multi-band imaging suffers from decreased SNR due to unfavorable coil geometry in cardiac applications. Our results show that regularized reconstructions reduce this noise amplification, albeit with increased inter-slice leakage, primarily from subcutaneous fat from the chest or back. To further reduce leakage, fat saturation can be applied as previously proposed for other T1-mapping methods12. Nonlinear reconstruction techniques with appropriate regularization can also be utilized for further removal of artifacts due to noise and leakage. However these were not explored in the current study.
Acknowledgements
The project described was supported by NIH R00HL111410 and NIH P41EB015894.References
1. Schelbert EB, Messroghli DR. "State of the Art: Clinical Applications of Cardiac T1 Mapping." Radiology. 2016 Mar;278(3):658-76.
2. Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB; Society for Cardiovascular Magnetic Resonance Imaging; Cardiovascular Magnetic Resonance Working Group of the European Society of Cardiology. "Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement." J Cardiovasc Magn Reson. 2013 Oct 14;15:92.
3. Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. "Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging." Magn Reson Med. 2005 Mar;53(3):684-91.
4. Ugurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, Lenglet C, Wu X, Schmitter S, Van de Moortele PF, Strupp J, Sapiro G, De Martino F, Wang D, Harel N, Garwood M, Chen L, Feinberg DA, Smith SM, Miller KL, Sotiropoulos SN, Jbabdi S, Andersson JL, Behrens TE, Glasser MF, Van Essen DC, Yacoub E; WU-Minn HCP Consortium. “Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project.” Neuroimage. 2013 Oct 15;80:80-104.
5. Stäb D, Wech T, Breuer FA, Weng AM, Ritter CO, Hahn D, Köstler H. "High resolution myocardial first-pass perfusion imaging with extended anatomic coverage." J Magn Reson Imaging. 2014 Jun;39(6):1575-87.
6. Weingärtner S, Akçakaya M, Basha T, Kissinger KV, Goddu B, Berg S, Manning WJ, Nezafat R. "Combined saturation/inversion recovery sequences for improved evaluation of scar and diffuse fibrosis in patients with arrhythmia or heart rate variability." Magn Reson Med. 2014 Mar;71(3):1024-34.
7. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. "Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty." Magn Reson Med. 2012 May;67(5):1210-24.
8. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. "Generalized autocalibrating partially parallel acquisitions (GRAPPA)." Magn Reson Med. 2002 Jun;47(6):1202-10.
9. Pruessmann KP, Weiger M, Börnert P, Boesiger P. "Advances in sensitivity encoding with arbitrary k-space trajectories." Magn Reson Med. 2001 Oct;46(4):638-51.
10. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. "SENSE: sensitivity encoding for fast MRI." Magn Reson Med. 1999 Nov;42(5):952-62.
11. Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, Yacoub E, Ugurbil K. "Evaluation of slice accelerations using multiband echo planar imaging at 3 T." Neuroimage. 2013 Dec;83:991-1001.
12. Peters DC, Thorn SL, Bregazi A, Hawley C, Stacy MR and Sinusas AJ. "Towards high-resolution fat-suppressed T1-mapping of atrial fibrosis in the left atrium: a fit-free three-point method." J Cardiov Magn Reson. 2015;17(Suppl 1):W17
Figures