0031
Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging1Radiology, NYU School of Medicine, New York, NY, United States, 2Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, NYU School of Medicine, New York, NY, United States
Synopsis
Fat-suppressed T1-weighted gradient-echo imaging is commonly used for abdominal MR examination. However, image quality can be compromised by inhomogeneous fat suppression and imperfect breath-holding. To overcome both limitations, we describe a novel technique for free-breathing fat/water separation (Dixon-RAVE).
Motion-robust acquisition is achieved by using radial sampling. A model-based reconstruction, which incorporates compressed sensing, parallel imaging, and fat deblurring, is used to obtain fat and water maps. Two extensions are described that enable motion-resolved fat/water separation (XD-Dixon-RAVE) and dynamic contrast-enhanced fat/water separation (DCE-Dixon-RAVE). The technique is demonstrated for various clinical applications, including free-breathing liver and breast exams in volunteers and patients.
Purpose
Fat-suppressed T1-weighted gradient-echo imaging is one of the most commonly used sequences in clinical practice and applied for various applications. However, spectral fat suppression typically fails in regions with strong field inhomogeneities. Furthermore, additional non-fat-suppressed T1-weighted scans need to be acquired to identify fat-containing lesions or to detect fat infiltration of organs.
Dixon methods have emerged as a valuable alternative, achieving improved robustness to B0 inhomogeneities. In addition, they provide water, fat, in-phase and opposed-phase images from a single scan. However, because conventional Dixon imaging relies on motion-sensitive Cartesian sampling, abdominal scans must be performed during breath-holding. This can be problematic for sick and elderly patients, and is impossible for sedated pediatric subjects.
To overcome these limitations, we describe a novel technique for free-breathing fat/water separation called “Dixon-RAVE”. By using a radial trajectory for data acquisition, motion-robust imaging becomes possible. The reconstruction procedure is based on a model-based approach, which accounts for the off-resonance frequency of fat and removes the blurring normally seen with radial sampling. Furthermore, it integrates both compressed sensing and parallel imaging. Two extensions are presented: To further improve motion robustness, respiratory information is extracted and used to reconstruct different motion states (XD-Dixon-RAVE). The second extension enables fat-water-separated dynamic contrast-enhanced measurements (DCE-Dixon-RAVE).
Methods
Sequence design
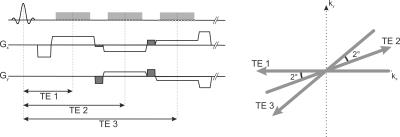
A T1-weighted radial 3D gradient-echo sequence with stack-of-stars trajectory is used for data acquisition1. Radial sampling is used in-plane according to the golden-angle scheme, while the slice direction is acquired with Cartesian phase-encoding. Three echoes are sampled within each repetition time using a bipolar readout. To improve k-space coverage, blip gradients are switched between the readout events, which rotate subsequent projections by 2 degrees. The entire acquisition scheme is shown in Figure 1.
Image reconstruction
Water ($$$W$$$) and fat ($$$F$$$) maps are estimated directly from the acquired k-space data $$$y$$$ by solving the following optimization problem:
$$\text{argmin}\sum_{c,t}\|E(W,F,\Phi)_{c,t}-y_{c,t}\|_2^2+\lambda_W\|\text{S}(W)\|_1+\lambda_F\|\text{S}(F)\|_1$$
Compressed sensing can be included by using L1-normalization with a sparsifying transform $$$S$$$. $$$E$$$ is the forward operator that synthesizes k-space data from the to-be-estimated water, fat, and B0 field maps ($$$\Phi$$$) and can be expressed as:
$$E(W,F,\Phi)_{c,t}=\text{FT}\left(C_c\cdot\exp\left(2\pi i\cdot\Phi\cdot t_n\right)\cdot W\right)+D(t)\cdot\text{FT}\left(C_c\cdot\exp\left(2\pi i\cdot\Phi\cdot t_n\right)\cdot F\right)$$
$$$FT$$$ performs a non-uniform fast Fourier transform and $$$t_n$$$ are the different echo times. Parallel imaging is incorporated via multiplication with the coil-sensitivity profiles $$$C_c$$$. $$$D(t)$$$ models the chemical shift in k-space2, which allows compensating for the off-resonant blurring of fat due to radial readout. This is achieved by using the exact readout time points $$$t=t_n+\tau_{n,k}$$$ of the samples $$$k$$$ of each spoke:
$$D(t)=\sum_{m=1}^6\alpha_m\cdot\exp\left(2\pi i\cdot\Delta f_m\cdot(t_n+\tau_{n,k})\right)$$ All reconstruction variants were implemented in Matlab (Mathworks, MA). The optimization problem was solved using either a Gauss-Newton3 or an lBFGS algorithm. The solvers were initialized with a precomputed field map created with either an unwrapping-based4 or a region-growing-based5 B0 mapping approach.
Extensions
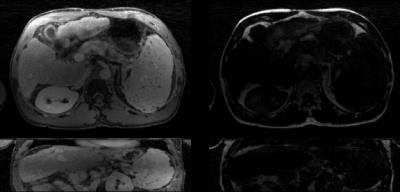
XD-Dixon-RAVE: While the underlying radial trajectory is inherently robust to motion, residual artifacts such as blurring or streaking can still appear. To further improve image quality, a respiratory signal is extracted from the k-space data. The acquired projections are then sorted into different respiratory phases using the signal. This enables reconstruction of respiratory-resolved images with finite differences as sparsifying transform along the temporal dimension6.
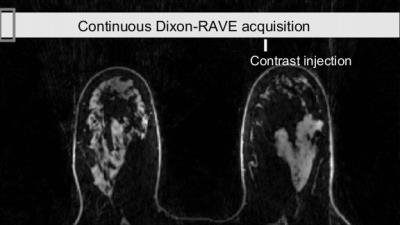
DCE-Dixon-RAVE: For fat-water-separated DCE imaging, data are acquired continuously during injection of a contrast agent. Afterwards, consecutive projections are grouped into frames. 4D time-resolved $$$W$$$ and $$$F$$$ maps are reconstructed using temporal total variation as regularizer.
Imaging studies
To demonstrate the broad applicability of the proposed technique, different IRB-approved examinations were performed during free breathing: (i) Liver scan of a volunteer (without contrast agent). (ii) Post-contrast liver scan of a 57-year-old patient. (iii) DCE breast scan of a 61-year-old cancer patient. Acquisition parameters and reconstruction details are shown in Table 1.
Results
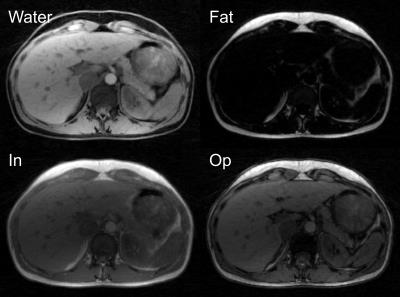
Figure 2 shows water and fat maps, as well as synthetically generated in-phase/opposed-phase images from the free-breathing volunteer scan. Results from the liver patient scan, reconstructed with XD-Dixon-RAVE, are shown in Figure 3. Respiratory motion is clearly resolved and sharp images with minimal blurring are obtained. Figure 4 shows the dynamic image series of the DCE breast scan, reconstructed with DCE-Dixon-RAVE.Discussion and Conclusion
By combining the concepts of 3D radial stack-of-stars sampling, model-based reconstruction, parallel imaging, and compressed sensing, Dixon-RAVE enables motion-robust fat/water-separated imaging during free-breathing. XD-Dixon-RAVE provides further reduction of residual motion artifacts by reconstructing motion-resolved fat/water maps. Fat-water-separated DCE imaging with high spatial and temporal resolution is possible with DCE-Dixon-RAVE.
In summary, the proposed fat/water separation framework promises high value for various clinical applications, especially for abdominal examination of patients with limited breath-holding capability.
Acknowledgements
1. Block KT et al., Towards Routine Clinical Use of Radial Stack-of-Stars 3D Gradient-Echo Sequences for Reducing Motion Sensitivity, JKSMRM 18:87-106 (2014)
2. Brodsky EK et al., Generalized k-Space Decomposition with Chemical Shift Correction for Non-Cartesian Water-Fat Imaging, Magn Reson Med 59:1151-64 (2008)
3. Doneva M et al., Compressed Sensing for Chemical Shift-Based Water-Fat Separation, Magn Reson Med 64:1749-59 (2010)
4. Liu J et al., Method for B0 off-resonance mapping by non-iterative correction of phase-errors (B0-NICE), Magn Reson Med 74:1177-88 (2015)
5. Berglund J et al., Three-Point Dixon Method Enables Whole-Body Water and Fat Imaging of Obese Subjects, Magn Reson Med 63:1659-68 (2010)
6. Feng L et al., XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing, Magn Reson Med 75:775-88 (2016)
References
Funding: NIH R01 EB018308, P41 EB017183
We acknowledge the use of field-mapping methods from the Fat-Water Toolbox (http://ismrm.org/workshops/FatWater12/data.htm) and other open-source contributions from the fat/water separation community.
Figures