0029
GABA and glutamate in children with Tourette Syndrome: a 7T 1H-MRS study1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University, Baltimore, MD, United States, 2FM Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Neuropsychology, Kennedy Krieger Institute, Baltimore, MD, United States, 4Department of Psychiatry and Behavioral Sciences, The Johns Hopkins University, Baltimore, MD, United States, 5Department of Neurology, The Johns Hopkins University, Baltimore, MD, United States
Synopsis
Studies have suggested that altered inhibition and excitation contribute to the pathology of Tourette syndrome, especially in cortical-striatal-thalamo-cortical (CSTC) pathways. GABA and glutamate were measured at 7T in large cohorts of healthy children and children with TS in regions of the CSTC network. GABA and glutamate were increased in the striatum. Glutamate was increased in the premotor region and correlated with reduced motor inhibition. These data support involvement of habitual behavioral pathways in TS. Historically the dopaminergic system has been considered to have a dominant role in TS; however, accumulating evidence strongly suggests involvement of GABA and glutamate neurotransmitter systems.
Purpose
To investigate GABA and glutamate alterations in the cortical-striatal-thalamo-cortical network in children with Tourette syndrome using 1H-MRS at 7 Tesla.Background
Tourette syndrome (TS), a common disorder in children and adolescents, is characterized by the presence of chronic, fluctuating motor and vocal tics. The underlying neurobiological basis for these tics is unknown, but are thought to involve cortical-striatal-thalamo-cortical pathways (CSTC)1. Glutamate acts as the primary excitatory neurotransmitter within the cortical, thalamic, and subthalamic nuclei. Reduced glutamate has been shown in several brain regions in TS and glutamate dysfunction in cortico-striatal afferents may support the generation of tic-like movements2. In contrast, GABA is the primary inhibitory neurotransmitter of striatal projection neurons, the globus pallidus, and interneurons within the cortex and striatum. Several studies have shown altered inhibitory function3 and changes in brain GABA levels4,5 (although the findings are variable) as well as reduced interneuron density in post-mortem tissue6. Furthermore, invasive studies have shown that blocking GABA leads to tic-like behaviors7. 1H-magnetic resonance spectroscopy at 7T allows separation of glutamate and glutamine, not available with 3T MRS.Materials and Methods
Participants: Thirty-two children diagnosed with TS (25M; mean age 9.9 years) and 43 typically developing children (8.1 years; 22M), were recruited (all between 5 – 12 yrs old). Parents or legal guardians of all participants provided informed, written consent and all participants provided assent prior to the study. Children had to have an observable tic score (TTS) above and were drug naïve or off medications for over 3 months prior to the study. Children with TS were excluded when they had secondary tics, significant medical illness, or other neurodevelopmental or psychiatric disorder except ADHD or ODD.
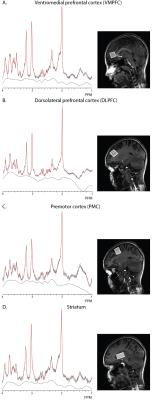
MRI and MRS Acquisition: MRS was performed using a 7T scanner (Achieva, Philips Healthcare, Best, The Netherlands) using a Nova Medical quadrature transmit head coil and 32-channel receive coil array. A T1-weighted MPRAGE structural brain image (TE/TR=2.1/4.8 ms; resolution: 0.6×0.6×0.6 mm3) was acquired for planning of the MRS voxel locations. Spectra were acquired from four ~8ml voxels: ventromedial prefrontal (VMPFC); dorsolateral prefrontal (DLPFC); premotor (PMC); and striatum (see figure 1) using the STEAM sequence with the following parameters: TE/TM/TR/NS = 14 ms/26 ms/3000 ms/96 averages and VAPOR water suppression.
Data analysis: LCModel8 was used to quantify both GABA and Glu using an in-house basis set that includes an acquired macromolecular basis spectrum. GABA, Glu, Gln, NAA and Cho, and were measured relative to water within each voxel. GABA/Glu was also calculated to investigate inhibitory-excitatory balance. Statistical analysis: A linear mixed-effects (LME) model with Fisher’s LSD as a post-hoc test was used to assess global metabolite differences across groups, controlling for age and sex. Regional differences were then tested using voxel specific LME models. Pearson- or partial correlations were used to assess the relation between symptom severity and metabolite levels.
Results
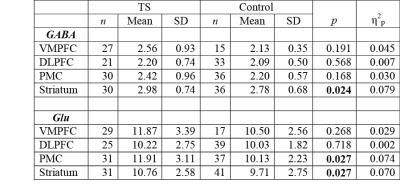
The 7T scan was well tolerated by all participants and example spectra are shown in Figure 1. Compared to typically developing controls, children with TS showed significantly increased GABA in the striatum (p=0.024), and increased glutamate within the striatum (p=0.027) and PMC (p=0.027) as shown in Table 1. No metabolite changes were present in the DLPFC, or VMPFC, regions. In the TS group only, increased PMC glutamate was significantly associated with reduced motor overflow (reflecting inhibitory control) on timed motor examination (r = -.52, p = 0.007). There were no significant findings with respect to the GABA/Glu ratio.Discussion and Conclusion
In this study, ultra-high-field (7T) 1H MRS was used to quantify concentrations of GABA and glutamate within CSTC pathways of young children with TS. Our results show that changes in GABA and glutamate were primarily observed in the striatum; no differences between the TS and control cohorts were identified in VMPFC and DLPFC. These results support the notion that abnormalities in the striatum play a major role in tics. Changes in other regions may relate to comorbidities. Furthermore our results showing increased levels of glutamate within both the PMC and striatum support involvement of habitual behavioral pathways.
The current study is the one of first investigation to obtain 7T MRS measurements of both glutamate and GABA within the striatum of TS children. While historically the dopaminergic system has long been considered to have a dominant role in TS; however, accumulating evidence strongly suggests involvement of other neurotransmitter systems. Understanding the role of these systems in key cortical regions may allow for a better understanding of the disorders as well as target future treatments.
Acknowledgements
This study was supported by the Tourette Association of America. This work was further supported by NIH P41 EB015909References
1. Singer HS. Treatment of tics and tourette syndrome. Curr Treat Options Neurol 2010;12:539-561.
2. Felling RJ, Singer HS. Neurobiology of tourette syndrome: current status and need for further investigation. J Neurosci 2011;31:12387-12395.
3. Gilbert DL. Motor cortex inhibitory function in Tourette syndrome, attention deficit disorder, and obsessive compulsive disorder: studies using transcranial magnetic stimulation. Adv Neurol 2006;99:107-114.
4. Puts NA, Harris AD, Crocetti D, et al. Reduced GABAergic inhibition and abnormal sensory symptoms in children with Tourette syndrome. J Neurophysiol 2015;114:808-817.
5. Draper A, Stephenson MC, Jackson GM, et al. Increased GABA contributes to enhanced control over motor excitability in Tourette syndrome. Curr Biol 2014;24:2343-2347.
6. Kalanithi PS, Zheng W, Kataoka Y, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A 2005;102:13307-13312.
7. Pogorelov V, Xu M, Smith HR, Buchanan GF, Pittenger C. Corticostriatal interactions in the generation of tic-like behaviors after local striatal disinhibition. Exp Neurol 2015;265:122-128.
8. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001;14:260-264.
Figures