0023
Individualized Tractography-Based Parcellation of the Globus Pallidus Pars Interna using 7T MRI in patients with Parkinson’s Disease Prior to DBS Surgery1CMRR / Radiology, University of Minnesota, Minneapolis, MN, United States, 2Neurology, University of Minnesota, Minneapolis, MN, United States
Synopsis
The success of deep brain stimulation (DBS) surgeries for Parkinson’s disease relies on the accurate placement of an electrode within the motor portion of subcortical brain targets. We use 7T MR-tractography to visualize the functional territories of the Globus Pallidus pars Interna. We found that the motor territory is located immediately posteromedially to the associative and limbic territories, akin to the subthalamic nucleus organization. This pattern was reproducible across two DBS patients. These findings shed new light on the functional organization of DBS targets, showing potential for providing valuable information to clinicians for targeting decisions and ultimately enhancing patient’s outcomes.
Introduction
The success of deep brain stimulation (DBS) surgeries for Parkinson’s disease (PD) relies on the accurate placement of an electrode within the motor portion of subcortical brain targets. Today’s targeting methods do not ensure such accuracy and it is estimated that 15-34% of DBS procedures require revisions1. We have previously demonstrated that ultra high-field (7 Tesla) MRI data is capable of generating reproducible, individualized parcellation of the subthalamic nucleus2 (STN) into functional territories. In this study, we demonstrate that such techniques can also be applied to the internal segment of the globus pallidus pars interna (GPi), another DBS target for the treatment of PD and dystonia. This study uses ultra-high field MRI to parcellate the GPi of PD patients prior to their DBS surgery with the goal of uncovering patient-specific functional territories of the GPi to aid DBS targeting.Methods
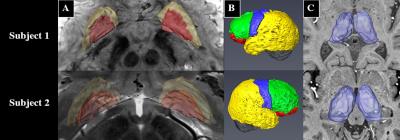
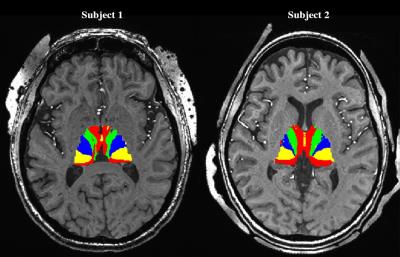
Prior to DBS surgery, two patients (1 male, 1 female) were imaged using a 7 Tesla MRI scanner. The protocol included a 0.6mm3 T1, a 0.4x0.4x1mm axial T2, a 0.4x0.4x0.8mm axial susceptibility-weighted imaging (SWI) and a 1.6mm3 DTI acquisition (whole brain, AP & PA directions, 54 directions -- including 4 B0, b = 1500s/mm2)2. The GPi was manually segmented on the T2 or the SWI image (Fig. 1A). The cortex was divided into limbic, associative, motor and “other” regions using a standard atlas2 and then brought to the individual T1 space2 (Fig. 1B). Many studies have shown that the GPi does not have direct connections to the cortex3,4,5; therefore, we executed the parcellation in two steps: 1) Identifying functional territories of the thalamus using tractography parcellation to the cortex, 2) parcellation of the GPi using the thalamic parcellations obtained in step 1 as targets. The thalamus was extracted from the Destrieux Atlas 20096 in T1 native space as obtained using the Human Connectome minimal preprocessing pipeline7. DTI preprocessing included motion, susceptibility artifact, and eddy current correction using topup8, three-fiber model estimation of the diffusion parameters using FSL’s bedpostX9. Tractography-based connectivity was computed at each voxel of the Thalamus (step 1) and the GPi (step 2) using FSL’s probtackX2. The tractography was performed unilaterally. To obtain the targets for the GPi parcellation, masks were derived based on the thalamus parcellation output files from step 1 (Fig. 2). Lastly, final electrode and contact location was extracted from a post-operative high-resolution computed tomography scan (0.6mm3) for one subject in order to assess clinical significance of our results.Results
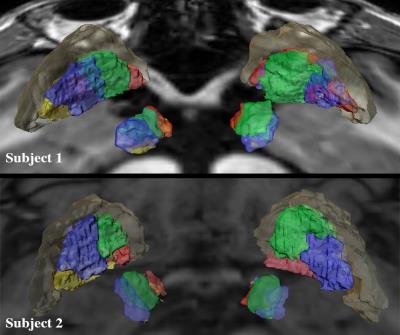
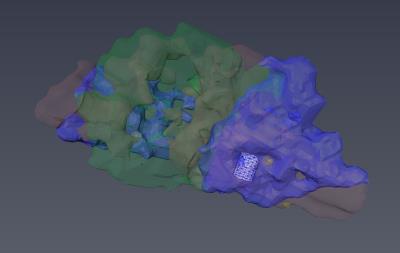
Successful parcellation of the thalamus is shown in Fig. 2 and serves two purposes; first, validation of the method following Behrens10 and Plantinga2 (step 1). Second, the identified functional regions of the thalamus were used as targets for parcellation of the GPi (step 2). Fig. 3 shows the parcellation of the GPi along with the parcellation of the STN for comparison using the methodology used in Plantinga2. A reproducible topographical functional organization of the GPi is seen in both patients. The motor territory is located posterolaterally in the parasagittal plane, or posteromedially along the GPi’s long axis. It is followed anteriorly by the associative and the limbic territories. A physiological validation of the imaging-based determination of the motor area was achieved by registering the post-op CT lead location with the anatomical model as shown for one patient in Fig. 4. The electrode was placed in the area we defined as the motor region. The clinically optimized DBS settings indicate that best motor improvement was seen with the active contact located in the motor region as indicated by the imaging-based model.Discussion
Thalamic parcellation obtained in step 1 validates our methodology as the estimated functional organization pattern is consistent with that reported in the literature10,2. The organization of the functional territories of the GPi observed here follows a similar pattern to that observed in the STN (Fig. 3). Our results are further validated by the post-operative electrode location and clinically optimized DBS programming settings. In fact, based on the imaging model, the most beneficial contact, as confirmed by improvement during DBS, was located in the motor region.Conclusion
Our results demonstrate the existence of multiple functional territories within the GPi. The observed GPi organization pattern is similar to that previously described in the STN2 and the thalamus2,10 and may reflect a common architecture of basal ganglia structures. These new findings provide a better understanding of the fundamental organization of the anatomical structures that are the targets for DBS surgery and neuromodulation therapy.Acknowledgements
This study was supported by the NIH R01-NS085188; P41 EB015894; P30 NS076408 and the University of Minnesota Udall center P50NS098573References
1. J.D. Rolston, et al. An unexpectedly high rate of revisions and removals in deep brain stimulation surgery: Analysis of multiple databases. Parkinsonism and Related Disorders. 2016; In Press.
2. Plantinga, B.R., et al. Individualized parcellation of the subthalamic nucleus in patients with Parkinson's disease with 7T MRI. NeuroImage. 2016; In Press.
3. Da Cunha, C., et al. Toward sophisticated basal ganglia neuromodulation: Review on basal ganglia deep brain stimulation. Neuroscience and Biobehavioral Reviews. 2015; 58:186-210.
4. Martinu, K. and Monchi, O. Cortico-Basal Ganglia and Cortico-Cerebellar Circuits in Parkinson’s Disease: Pathophysiology or Compensation? 2013; 127(2):222-236
5. Gunaydin, L.A and Kreitzer, A.C. Cortico-basal ganglia circuit function in psychiatric disease. Annual Review of Physiology. 2016; 78:324-350.
6. Destrieux, C. et al. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010; 53(1):1-15.
7. Glasser, M.F. et al. The minimal preprocessing pipelines for Human Connectome Project. NeuroImage. 2013; 80:105-124.
8. Andersson, J.L. et al. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage; 20:870-888.
9. Behrens T.E. et al. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage. 2007; 34:144-155.
10. Behrens T.E. et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003; 6:750-757.
Figures