0013
Interventional Magnetic Resonance Imaging Guided Carotid Embolectomy using a Novel MRI-Conditional Resonant Catheter: Demonstration of Preclinical Feasibility1Department of Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 2Penumbra Inc, Alameda, CA, United States
Synopsis
MR-guided endovascular interventions can provide real-time imaging biomarkers for procedures such as stroke embolectomy. The purpose of this study is to determine preclinical feasibility and efficacy of imaging wireless resonant circuits embedded in a MR compatible catheter system for in vivo MR-guided carotid embolectomy in porcine stroke models. The resonant catheter system performed effectively under real-time MRI with recanalization rates (TICI 2b/3) similar to reported clinical rates in stroke embolectomy. Qualitative physiologic measures of flow under MRI were comparable to those measured under X-ray, demonstrating feasibility of resonant catheter system for in vivo carotid occlusion and embolectomy under real-time MRI.
Introduction
Stroke is the leading cause of long-term disability and the fifth leading cause of death in the United States.1 Recent randomized controlled trials demonstrated clinical superiority of catheter-based endovascular embolectomy over medical therapy alone within six hours after stroke onset.2-4 Our goal is to improve stroke treatment further using real-time MRI guidance; thus allowing intra-procedural evaluation of brain parenchyma viability to direct reperfusion therapy at living and salvageable tissue. By application of single-loop wireless resonant circuits 5,6 for MR visualization with a MR compatible catheter, we aim to assess the visualization and efficacy of a resonant circuit catheter system for in vivo MRI-guided carotid embolectomy.Methods
Resonant Catheter Design: A custom MR compatible 6Fr catheter was constructed with a 0.00075” thick PTFE liner with a single continuous 0.005” diameter PEEK fiber wrapped circumferentially, and then heat sealed with a layer of PEBAX at 450° F. The RF resonator was constructed and sealed within the distal tip using a single insulated 30 gauge longitudinal copper loop (50 mm by 2.5 mm) soldered to a printed circuit board capacitor (figure 1), and tuned to the Larmor frequency of the 1.5 T MRI scanner (Phillips Achieva).
In vivo carotid embolectomy: In four farm pigs (40-45kg), the resonant catheter system was placed in either the left or right common carotid artery (CCA). Two industry standard clots 7 were soaked in 1% gadolinium solution and variable amounts were injected through the catheter under X-ray until adequate arterial occlusion was noted in a total of n=13 arteries. The arteries analyzed included the internal carotid (ICA), external carotid (ECA), and CCA. Catheter navigation and embolectomy were then performed under real-time MRI. Restoration of blood flow was confirmed via MR and X-ray imaging and graded by the Thrombolysis in Cerebral Infraction (TICI) 8 scale; TICI 2b/3 were considered successful recanalization.
Imaging Parameters: All studies were performed in a clinical hybrid interventional XMR suite combining a 1.5T clinical MR scanner and a C-arm DSA system, with a 32-channel cardiac coil and a real-time low flip angle bSSFP sequence or GRE sequence with typical flip angles of 5-20°, TE = 1.555 ms, TR = 4.972 ms, Slice Thickness = 10mm, Matrix = 216 × 218, FOV = 26 cm. Additionally, a contrast enhanced MR angiography sequence (CE-MRA), a non-contrast in-flow MRA, and DWI images were acquired at baseline, after clot occlusion, and immediately after each embolectomy.
Results
The surface loop coil was visible both on the sagittal bSSFP and coronal 5° GRE sequence (figure 2). Figure 3 demonstrates active catheter visualization pre-embolectomy and during aspiration with clot noted as filling defect in the catheter lumen. Of the 13 successfully occluded arteries, we were able to demonstrate successful recanalization in 11 by DSA analysis and 8 arteries by MRA (Figure 4). Baseline, post occlusion, and post embolectomy vasculature from a single carotid artery experiment are shown both by X-ray angiography and CE-MRA (figure 5). When compared with reported spontaneous recanalization of 17% in stroke patients 9, binomial tests indicated that the proportion of successful recanalization based on DSA of 0.85 (11 of 13) and MRA of 0.62 (8 of 13) were both significantly higher (p<0.001). Additionally, binomial testing comparing DSA (0.85) and MRA (0.62) to the clinical rates of success in stroke embolectomy (79.2%) 10 showed no significant difference with p=1.00 and p=0.16, respectively. Lastly, a Wilcoxon signed rank test indicated no significant difference between TICI scores analyzed by DSA and MRA (z=1.494, p=0.135).Discussion
The resonant marker aspiration catheter system is feasible for experimental carotid occlusion and embolectomy under real-time MRI in vivo, and qualitative physiologic measures of flow under MRI were similar to those measured under X-ray. Due to the swine biological model and robust collateral perfusion seen in the porcine brain, we did not expect transient carotid occlusion to cause regional infarct and significant DWI changes. In further studies, a longer period of time or more controllable clot formation may be necessary to detect diffusion changes for guiding intra-procedural decisions.Conclusion
With demonstrated visualization and compatibility under MRI, the catheter system performed effectively in preliminary in vivo endovascular embolectomy and is a step toward clinical endovascular interventional MRI.Acknowledgements
This work was supported by the National Institute of Biomedical Imaging and Bioengineering grants R01EB012031 and R21EB020283 and the National Center for Advancing Translational Sciences grant UL1TR000004. The authors thank Penumbra, Inc, for in-kind provision of catheter shafts, materials, and engineering expertise.References
1. Dariush Mozaffarian, Emelia J. Benjamin, Alan S. Go, et al. Heart Disease and Stroke Statistics – 2016 Update. American Heart Association. 2016;133: e38-e360
2. Berkhemer O, Fransen P, Beumer D, et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. New Engl J Med. 2014;372(1):11–20.
3. Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N Engl J Med. 2015;372(11):1009–18.
4. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30.
5. Kaiser, M, Detert M, Rube MA, et al. Resonant marker design and fabrication techniques for device visualization during interventional magnetic resonance imaging. Biomed Eng / Biomed Tech. 2015 Jan 1;60(2): 89-103.
6. Quick HH, Zenge MO, Kuehl H, et al. Interventional magnetic resonance angiography with no strings attached: Wireless active catheter visualization. Magn Reson Med. 2005;53(2):446–55.
7. Duffy S, Farrel M, McArdle K, et al. Novel methodology to replicate clot analogs with diverse composition in acute ischemic stroke. J NeuroIntervent Surg 2016;0: 1-7.
8. Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109 –137
9. Kassem-Moussa H, Graffagnino C. Nonocclusion and Spontaneous Recanalization Rates in Acute Ischemic Stroke – A Review of Cerebral Angiography Studies. Arch Neurol. 2002;59(12): 1870-1873.
10. Pereira VM, Gralla J, Davalos A, et al. Prospective, Multicenter, Single-Arm Study of Mechanical Thrombectomy Using Solitaire Flow Restoration in Acute Ischemic Stroke. Stroke. 2013;44: 2802-2807.
Figures
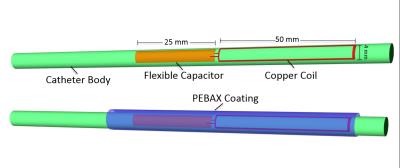
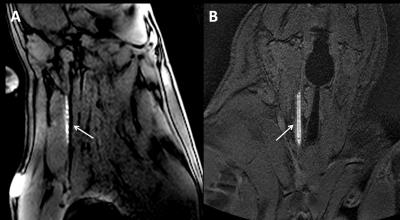
Figure 2: Demonstration of resonant catheter system visualization during active image tracking and across each orientation of navigational scan. A) BSSFP (TE = 2 ms, TR = 3.9 ms, Flip angle = 10°, Slice Thickness = 8mm, Matrix = 216 × 218, FOV = 26 cm) B) GRE (TE = 1.7 ms, TR = 5.3 ms, Flip angle = 5°, Slice Thickness = 5mm, Matrix = 216 × 218, FOV = 26 cm)
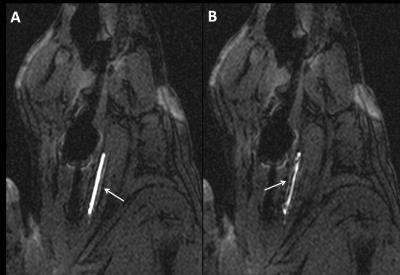
Figure 3: Active image tracking under BSSFP sequence during A) navigation to occluded carotid artery and B) clot aspiration into lumen of catheter, noted as filling defect.
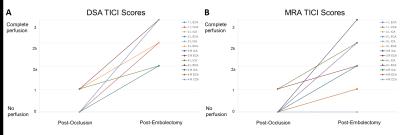
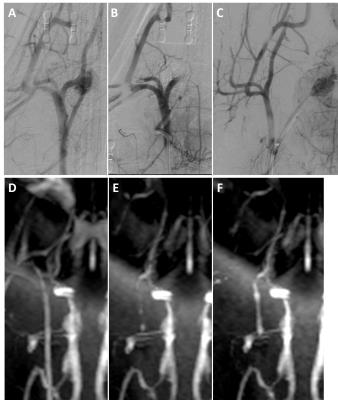
Figure 5 - Right carotid vasculature from a single experiment demonstrated via DSA (above) and MRA (below) at (A,D) baseline start of study, (B,E) post clot occlusion of carotid vasculature, and (C,F) post embolectomy through manual aspiration.