0005
A Combined 7 Tesla MRI/NMR Probe Head for Photochemical Applications.1Otto Diels Institute for Organic Chemistry, Kiel University, Kiel, Germany, 2Molecular Imaging North Competence Center, University Medical Center Schleswig-Holstein, Kiel, Germany, 3Institute of Physical and Theoretical Chemistry, Graz University of Technology, Graz, Austria, 4Medical Physics in Radiology, German Cancer Research Center, Heidelberg, Germany
Synopsis
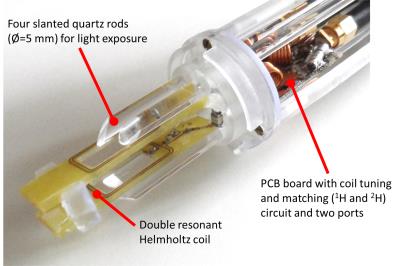
The development of new photoswitchable contrast agents requires analysis with both NMR and MRI. In this work a combined probe head for in situ light exposure is presented which can be used on NMR and MRI systems with both 7 Tesla. A probe head with four slanted quartz rods was fabricated. A dual tuned (1H and 2H) Helmholtz coil was integrated into the probe head. NMR and MRI experiments were performed on photoswitchable solutions (e.g. contrast agents). Results show that photoswitchable solutions can be successfully switched in situ and simultaneously analyzed with NMR or imaged with MRI.
Introduction & Purpose
The development of novel photoswitchable MRI contrast agents1,2 (CA) requires analysis with both NMR and MRI. Due to limited space in small bore NMR probe heads samples are usually exposed to light outside the NMR system3 preventing the spectroscopy of samples with short half-lifes. Exposure is also performed via sand blasted light fibers in coaxial NMR tube inserts4 inside the NMR system prohibiting sample spinning thus leading to long exposure times. In wide bore NMR systems light exposure via a slanted quartz rod was presented5 enabling sample spinning but requiring coaxial inserts for homogeneous light exposure. MR imaging of small NMR sample tubes is tedious since imaging is usually performed in larger coils requiring bigger sample volumes.
In this study a small bore NMR probe head with four slanted quartz rods for homogenous light exposure is presented which can be used for NMR and MR imaging experiments in systems with both 7 Tesla.
Methods
A rectangular (12 mm×20 mm) Helmholtz coil with eight windings in total was constructed on a double layer PCB board. A window was milled into the PCB enabling light exposure through the coil center. Both parts of the coil were fixed in a distance of 6 mm to fit a standard NMR tube with a diameter of Ø=5 mm. For NMR experiments the coil was tuned to 1H and 2H (for locking at the NMR system) at 7 Tesla with f1H(NMR)= 300.02 MHz and f2H= 46.05 MHz respectively and matched to 50 Ω. A TxRx-switch for the 7T MRI was built including a modified pre-amplifier from a 3T MRI system (Magnetom TIM Trio, Siemens Healthcare, Erlangen, Germany) tuned to a slightly higher frequency of f1H(MRI)= 300.42 MHz. For MRI experiments the coil was tuned to the MRI 1H frequency. Coil, tuning/matching circuit and four slanted quartz rods side were integrated into a holder (Fig. 1) fabricated using a 3D stereolithography printer (Form 2, Formlabs Inc., Somerville, USA). As light sources LEDs with different emitting wavelengths were used. A sheet of high-reflective PTFE was integrated in the probe head shielding to reflect light not targeting the sample tube.
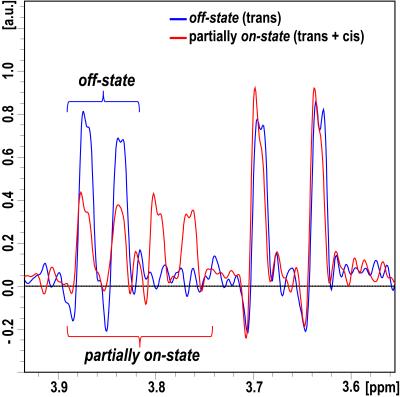
NMR experiments were performed on a 300 MHz spectrometer (ARX 300, Bruker, Ettlingen, Germany). NMR spectra were acquired from a photoswitchable solution (here: enzyme inhibitor; 3.5 mg in 5 ml in deuterated Dimethyl sulfoxide (DMSO)) in an NMR tube at room temperature while spinning the sample. A reference spectrum was recorded with the solution in off-state. A second spectrum was acquired after 10 min in situ light exposure with 440 nm (solution in on-state). All data was collected via an FID sequence with following parameters: FA=90°, AQ-window=0.8 s, TR=1.8 s, 128 averages, TA=4 min.
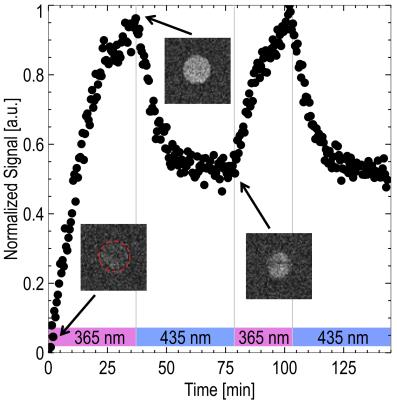
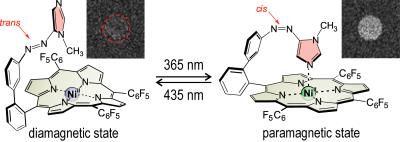
MRI experiments were performed on a 7 Tesla small animal MRI system (ClinScan 7T, Bruker) with a photoswitchable CA2 consisting of 2 mM Nickel(II)-porphyrin with Azo-N-methylimidazole ligand in 5 ml DMSO at room temperature. For maximum contrast enhancement a 2D inversion recovery (IR) TrueFISP sequence with following parameters was used: TE=1.4 ms, TR=15 s, TI=1.1 s, SL=2 mm, Mtx=128×128, FoV=20×20 mm², 300 repetitions. Inversion time was set to the zero signal for the solution in diamagnetic state. While imaging the solution was switched to the paramagnetic state in situ with exposure of 365 nm light. After illumination a second IR sequence was performed to identify the TI of the on-state CA solution. Switching to the diamagnetic state was performed with 435 nm light.
Results
Figure 2 shows the acquired NMR spectra (clipped) of the reference and exposed solution. Due to the short half-life of about 14 min the solution is not switched completely into the on-state showing residual signal of the off-state. The effect of photoswitching can clearly be determined. The lock for shimming via the 2H channel was performed automatically by the NMR system.
Figure 3 shows two switching cycles of the MRI imaging experiments with the photoswitchable CA. Signal enhancement is generated by switching the CA from diamagnetic into paramagnetic state (Fig. 4). The resulting T1 reduction was found to be 230 ms.
Discussion & Conclusion
In this work a combined 7 Tesla MRI/NMR probe head was presented. It proved to be well suitable for either NMR or MRI experiments. These preliminary results show that photoswitching can be fully performed inside an NMR and MRI system without removing the sample. The presented probe head is beneficial for the development of photoswitchable CAs featuring shorter switching times. In future switching experiments will be performed by triggering the light source via the NMR/MRI console which can be performed unattended.Acknowledgements
The authors would like to thank Holger Franzen from the spectroscopy department of Kiel University for the help with acquiring and analyzing spectra. This work was funded by the “Deutsche Forschungsgemeinschaft” (DFG) via the collaborative research center 677 (SFB 677) “Function by Switching”.
References
1. Venkataramani S, Jana U, Dommaschk M, Sönnichsen FD, Tuczek F, Herges R. Magnetic Bistability of Molecules in Homogeneous Solution at Room Temperature. Science 2011;331:445-448.
2. Heitmann G, Schütt C, Gröbner J, Huber L, Herges R. Azoimidazole Functionalized Ni-Porphyrins for Molecular Spin Switching and Light Responsive MRI Contrast Agents. Dalton Trans. 2016;45:11407-11412.
3. Mills A, O'Rourke C. Photocatalytic Organic Synthesis in an NMR tube: C-C coupling of Phenoxyacetic Acid and Acrylamide. Catal. Today 2014;230:256-264.
4. Nagashima T, Ueda K, Nishimura C, Yamazaki T. Structure-Correlation NMR Spectroscopy for Macromolecules Using Repeated Bidirectional Photoisomerization of Azobenzene. Anal. Chem. 2015;87:11544-11552.
5. Stadler E, Eibel A, Neshchadin D, Gescheidt G. Toward Matching Optically and NMR Active Volumes for Optimizing the Observation of Photo-Induced Reactions by NMR. Z. Phys. Chem. 2016; DOI: 10.1515/zpch-2016-0854
Figures