0004
Cardiac Synchronization at Ultra-High Field Using a 3-Lead ECG Trigger Device1The Centre for Advanced Imaging, The University of Queensland, Brisbane, Australia, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Siemens Healthcare Pty Ltd, Brisbane, Australia, 4Richard Slaughter Centre of Excellence in CVMRI, The Prince Charles Hospital, Brisbane, Australia
Synopsis
Accurate cardiac synchronization is essential in CMR. At ultra-high field, ECG triggering can be significantly impacted by the magneto-hydrodynamic (MHD) effect. Here, we investigate the performance of a conventional 3-lead ECG trigger device and a state-of-the-art trigger algorithm for cardiac ECG synchronization at 7 T. We show that by appropriate subject preparation and by including a learning phase for the R-wave detection outside of the magnetic field, reliable ECG triggering at ultra-high field is feasible despite severe distortions of the ECG signal. A quantitative analysis in 10 healthy subjects revealed a trigger sensitivity and specificity of 97.6% and 98.4%, respectively.
Background
High spatial resolution and new opportunities for MR-based tissue characterization or metabolic imaging have recently encouraged investigations into ultra-high-field (B0 ≥ 7 Tesla) cardiovascular magnetic resonance (CMR). While accurate cardiac synchronization is essential, ECG triggering at ultra-high field can be significantly impacted by the magneto-hydrodynamic (MHD) effect. Blood flow within the static magnetic field induces a voltage, which superimposes the ECG signal and can affect the recognition of the R-wave. Scaling with B0, the effect is particularly pronounced at ultra-high field (1-3).
The purpose of this study was to fully explore the technical capabilities of a 7 T research MR scanner and state-of-the-art 3-lead ECG equipment for cardiac synchronization at ultra-high field. We demonstrate that by including an appropriate ECG learning phase outside of the magnetic field, existing ECG trigger technology in 7 T research systems allows for generating stable and reliable ECG trigger signals in healthy volunteers.
Methods
CMR examinations were performed in 10 healthy volunteers on a non-commercial 7 T whole-body research MRI scanner (Siemens Healthcare, Erlangen, Germany) under institutional review board permission. The system was equipped with a 3-lead ECG trigger device (Siemens Healthcare, Erlangen, Germany) and a state-of-the-art trigger algorithm (4-5) for cardiac synchronization. An 8-channel Tx/32-channel Rx cardiac array (MRI Tools GmbH, Berlin, Germany), operated in single-channel transmit mode, was used for RF transmission and signal reception.
To ensure accurate triggering, ECG electrodes were placed following the manufacturer’s instructions, and in conjunction with a senior electrophysiology cardiac scientist. The trigger algorithm was calibrated by observing the subject’s ECG outside the magnet for approximately 30s (1,6). This learning phase allows the trigger algorithm to store the shape of the rising edge of the R-wave without the presence of the MHD effect. Once learning is completed, the trigger algorithm continuously compares the incoming ECG signal to the learned shape in real-time and initiates trigger events based on several conditions (4).
Cine CMR was performed after 3rd-order B0 shimming using a high-resolution breath-held ECG-retro-gated segmented two-dimensional spoiled gradient echo sequence with the following acquisition parameters: field of view (FOV) = 360 x 270 mm², matrix = 352 x 264, slice thickness = 6.0 mm, echo time (TE) = 3.13, repetition time (TR) = 6.1 ms, receiver bandwidth (BWRX) = 592 Hz/px, flip angle = 60 deg, phases = 20, temporal resolution = 64 ms, parallel imaging (GRAPPA, R = 3). To assess the performance of the underlying trigger setup, ECG trigger signals and trigger events were recorded. Vectorcardiograms were generated and analyzed qualitatively.
To obtain a quantitative estimate, false negative (unidentified R-wave) and false positive (trigger not identifying an R-wave) trigger events were identified manually in the ECG recordings. From each subject, a representative continuous section of the ECG signal containing up to 500 trigger events was included in the evaluation. From the results, sensitivity and specificity were calculated as follows:
Sensitivity = (NRR – NFN) / NRR,
Specificity = (NRR – NFP) / NRR,
with NRR, NFP and NFP denoting the number of RR-intervals, false negatives and false positives, respectively.
Results
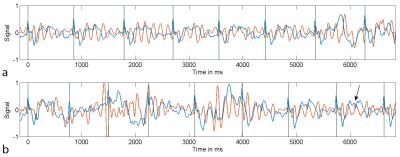
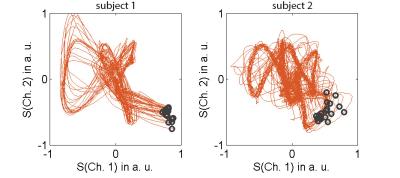
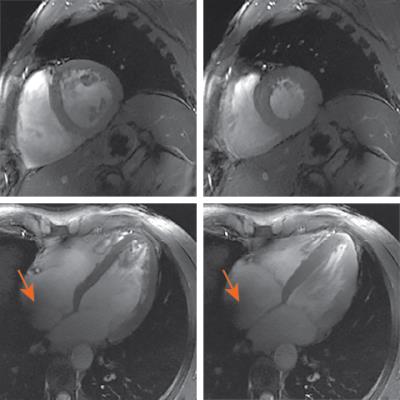
A representative section of the recorded ECG signal time curves is shown in Fig. 1a. Despite severe MHD-based signal distortions, trigger events are typically initiated accurately. Increased distortions are particularly visible during periods of enhanced breathing in preparation for a breath-held scan (Fig. 1b). Examples of typical vectorcardiograms are given in Fig. 2. Only little variation is observed in the location of the trigger events, indicating a high trigger fidelity. Imaging gradients did not significantly impact the ECG signal (6). For the initial quantitative evaluation in 10 volunteers, an overall of 4634 R-waves were investigated, yielding 113 false negative (sensitivity = 97.6%) and 76 false positive (specificity: 98.4%) events. For the vast majority of cases, the reconstructed images were free of visible trigger-related artefacts. Examples are given in Fig. 3.Conclusion
Our results indicate that reliable cardiac ECG triggering is feasible in healthy volunteers at ultra-high field utilizing a state-of-the-art 3-lead trigger device. By means of accurate subject preparation and by including a learning phase outside of the magnetic field, the employed trigger algorithm provided sufficient accuracy for high-fidelity CMR despite severe ECG signal distortions by the MHD effect. Future work will need to evaluate the synchronization setup in larger cohorts and patients with cardiac arrhythmia. Apart from CMR, also other ultra-high-field imaging applications such as human brain functional MRI with physiologic noise correction may benefit from robust ECG triggering and the easy instrumentational setup.Acknowledgements
MB acknowledges funding from ARC Future Fellowship grant FT140100865. The authors acknowledge the facilities of the National Imaging Facility at the Centre for Advanced Imaging, University of Queensland.References
1.
Suttie, NMR Biomed 2012;25:27–34.
2.
Frauenrath, J Cardiovasc Magn Reson 2010;12:67.
3.
Krug, J Cardiovasc Magn Reson 2013;15:W19.
4.
Frank, US patent application 20100191134 A1. July 2010.
5.
Knesewitsch, J Cardiovasc Magn Reson 2013;15:3.
6. Stäb, Tomography 2016;2:167-174.
Figures