4965
Optimal Strategies for CSF and Tissue Suppression in Velocity-Selective Arterial Spin Labeling1Department of Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 2University of Wisconsin-Madison, Madison, WI, United States, 3Department of Radiology, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
Velocity-selective arterial spin labeling (VS-ASL) inherently suffers from low signal-to-noise ratio (SNR) and contamination from cerebrospinal fluid (CSF) motion. This study aims to develop and evaluate optimal strategies for inversion based background suppression (BGS). Specifically, we investigate the influence of the timing of signal nulling and inflow from outside the region of interest. Our results suggest an optimized BGS which allows VS-ASL based measurement of cerebral blood flow maps with reduced CSF contamination while preserving sufficient perfusion signal.
Purpose
Velocity-selective arterial spin labeling (VS-ASL) is robust to quantification error in cerebral blood flow from transit time delays but inherently suffers from poor signal-to-noise ratio (SNR).1 Apparent SNR can be improved using background suppression (BGS) inversion pulses and optimal strategies for BGS have been proposed for pseudocontinuous ASL (PC-ASL).2 However, VS-ASL has differing demands of BGS due to sensitivity to cerebrospinal fluid (CSF) flow3,4 and methods for VS-ASL-BGS are under investigated and under reported. This study investigates optimization strategies for BGS in VS-ASL.Methods
VS-ASL strategies:
Figure 1a shows VS-ASL with BGS timings aimed at minimizing the longitudinal magnetization (MZ) of target tissues only considering MZ at the start of image acquisition. This is similar to past PC-ASL optimization strategies.2 Figure 1b shows proposed VS-ASL with BGS timings aimed for multiple criteria: (1) minimize MZ,GM and MZ,WM at TREAD to limit noise from fluctuations in static tissues; (2) minimize MZ,CSF at both TTAG and TREAD to limit noise from slow moving spins and pulsations; (3) maximize MZ,Blood at TTAG to maximize the detectability of perfusion signals. Further, the proposed BGS optionally considers the inflow of blood between BGS pulses and the use of selective pulses to increase SNR. A weighted MZ,Blood (MZ,Blood,w) was introduced to account for spins that did not experienced the full BGS scheme, as shown in Figure 2. BGS satisfying these criteria was solved for using Bloch simulation.
Image acquisition of normal volunteers:
Six healthy subjects were included in this IRB approval study using a 3T scanner (GE Signa Premier) with a 48ch coil. Acquisition parameters include: BIR-85 VS preparation with velocity cutoff (VC) = 1.09 cm/s, with fat saturation and vessel suppressing immediately before the imaging module. Three sets of VS-ASL BGS scans were acquired: (1) BGS scheme with one pre-VS inversion and one post-VS inversion with timing selected at 2s and 4.89s (flow-sensitive [FS] VS-ASL, Figure 1b), (2) proposed BGS scheme with nonselective pre-VS inversion (NS VS-ASL), and (3) proposed BGS scheme with selective pre-VS inversion (Sel VS-ASL). A multi-delay pseudo-continuous ASL (MD-PC-ASL) scan was acquired as reference standard. A T1-weighted scan was also acquired for anatomical reference.
Image analysis:
VS-ASL and T1-weighted images were spatially registered to MD-PC-ASL images with normalized mutual information by referencing to its PD images. GM, WM, and CSF masks were calculated from T1-weighted images. Perfusion signal and noise level were estimated from the magnetization difference (ΔM) maps from the first two control/tag pairs. A mean ΔM map was calculated by averaging the ΔM maps from the first two repetitions. Signal level for each ASL scan was extracted from the mean value in each binary mask from normalized difference images. Mask noise values were calculated from the subtraction of two ΔM maps acquired back to back.
Results
The BGS scheme was found to have an optimal solution when the three inversion pulses were placed at 4.5s, 6.8s, and 7.4s, with VS tag at 6.65s. MZ,CSF and MZ,Blood from Bloch simulation are shown in Figure 2. The proposed background suppression MZ,CSF was found to be 0.98% at TTAG and 0.91% at TREAD. The MZ,Blood that experienced full BGS scheme (NS VS-ASL) was found to be 47.9% at TTAG, whereas the MZ,Blood,w that experienced partial BGS scheme (selective VS-ASL) was found to be 63.5% at TTAG. ΔM maps showed comparable GM and WM contrast among the four acquisitions (Figure 3). The FS VS-ASL showed considerably higher signal surrounding the brain stem. The overall measured perfusion signal was higher with selective BGS VS-ASL than the ones measured with nonselective BGS VS-ASL. Results from signal and noise analysis agreed with visual evaluation (Figure 4). The signal level in FS VS-ASL was found to have the highest perfusion signal and noise level in CSF among the three BGS schemes.Discussion and conclusions
VS-ASL background suppression accounting for magnetization at both tag time and readout allows for blood flow estimates without CSF contamination. This comes at the penalty of a lower SNR, which can be partially compensated by using spatially selective inversion pulses. The sequence also has lower time efficiency, for which further optimization may be possible using T2 sensitive BGS.6 Future investigation is needed to characterize and compare the proposed VS-ASL to MD-ASL in elderly and diseased populations were differences may arise.Acknowledgements
The authors thank GE Healthcare for their research support and NIH support from NS066982References
- Wong EC, et al. Velocity-selective arterial spin labeling. Magn Reson Med. 2006;55:1334-1341.
- Maleki N, et al. Optimization of background suppression for arterial spin labeling perfusion imaging. Magn Reson Mater Phy. 2012;25:127-133.
- Liu D, et al. Quantitative measurement of cerebral blood volume using velocity-selective pulse trains. Magn Reson Med. 2017; 77: 92–101.
- Wu WC, et al. Intravascular effect in velocity-selective arterial spin labeling: the choice of inflow time and cutoff velocity. Neuroimage. 2006;32:122-128.
- Guo J, et al. An optimized design to reduce Eddy current sensitivity in velocity-selective arterial spin labeling using symmetric BIR-8 pulses. Magn Reson Med 2015;73:1085-1094
- Wong EC. Time efficient CSF suppressed velocity selective ASL using a T2-FLAIR preparation. Proc. Intl. Soc. Mag. Reson. Med. 2004.
Figures

Figure 1. VS-ASL pulse sequence diagram for FS BGS scheme and the BGS scheme for optimization.
The BGS scheme for optimization utilized one pre-VS inversion pulse implemented before VS tag and two inversion pulses inserted during the fixed PLD (a). The flow-sensitive BGS scheme utilized one pre-VS nonselective inversion pulse and one post-VS nonselective inversion pulse (b). GZ, gradient in z direction; PLD, postlabeling delay; TTAG, the time immediately before VS labeling; TREAD, the time immediately before imaging.

Figure 2. Bloch simulations of longitudinal magnetization time courses in CSF and blood.
Time course of CSF (MZ,CSF) when it has been minimized at the time immediately before VS tag and imaging readout (a). Time course of blood (MZ,Blood) when optimized to have sufficient contrast between tag and control at the time immediately before VS tag as nonselective (b) and selective (c,d) inversion BGS were included. Time course of blood that experienced only partial BGS was used for calculating a weighted MZ of blood to account inflow effects from the selective inversion pulse. MZ,Blood,NonSat, MZ,Blood only experienced inversion; MZ,Blood,NonInv, MZ,Blood maintained equilibrium magnetization.

Figure 3. Magnetization difference maps from a healthy volunteer.
Magnetization difference maps were obtained from a MP-PC-ASL scan and three VS-ASL scans with different BGS modules. The difference maps showed comparable image contrast and sufficient signal-to-noise ratio among the four scans. Discernable bright signals (yellow arrows) were observed in the ventricles when performing FS VS-ASL. With the proposed BGS module, signals from CSF were suppressed and therefore reduced noise from CSF pulsation. By alternating spatial selection of inversion pulse before VS tag, noticeable globally signal improvement was found when selective pulse was applied.
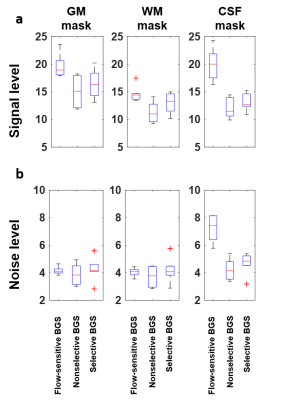
Figure 4. Signal level and noise analysis of difference maps.
Boxplots of the signal levels from difference images revealed that cerebrospinal fluid contamination was considerably greater in FS VS-ASL (a). Results from gray and white matter had comparable noise fluctuations, which revealed equivalent stability of proposed BGS scheme (b). Lower signal levels were observed with the proposed BGS scheme, potentially due to the inclusion of additional inversion pulses. GM, gray matter; WM, white matter mask; CSF, cerebrospinal fluid mask; BGS, background suppression.