4925
Characterization of Bias in Quantitative Susceptibility Mapping with Anisotropic Imaging Resolution: Studies in a Numerical Phantom, 3D Printed Liver Phantom, and In Vivo Patient Scans1Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 4Medicine, University of Wisconsin-Madison, Madison, WI, United States, 5Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Quantitative susceptibility mapping (QSM) is a promising technique for measuring iron concentration in patients with liver iron overload. In liver QSM, the constraints of scan time in a breadth-hold and the requirement of a short first echo time lead to limited imaging resolution, with anisotropic voxels. In this work, we characterized bias in liver QSM with anisotropic imaging resolution in simulation, a 3D printed liver phantom and patients. Our study shows that resolution-induced bias is related to the downsampling direction and is spatially-varying. In vivo results suggest the liver imaging resolution along the left-right dimension may affect liver susceptibility measurements.
Introduction
Quantitative susceptibility mapping (QSM) is a promising technique for non-invasive quantification of liver iron concentration (LIC) in patients with iron overload1. In liver QSM, 3D multi-echo images are obtained in a single breath-hold with a short first echo time2 to reduce respiratory motion and measure the broad range of LIC, respectively. The need for a short scan time limits imaging resolution along the phase-encoding directions, whereas the need for short echo times limits resolution along the readout direction. In practice, anisotropic imaging resolution is typically obtained. QSM estimates have shown bias in low resolution imaging of phantoms3 and the brain4. However, the effect of anisotropic imaging resolution has not been characterized in the liver which has a complex shape and is surrounded by other organs with varying susceptibilities. The purpose of this work was to characterize the bias in liver QSM with anisotropic imaging resolution.Methods
Simulation: A numerical abdominal phantom was created by segmenting a high-resolution patient MR image and assigning realistic 1.5T MRI properties to each tissue (Table 1). To assess the effect of liver orientation relative to the main magnetic field, the phantom was evaluated in two positions, i.e., supine position (“phantom-0°”) and 90° rotated in the coronal plane (“phantom-90°”). For each position, 3D multi-echo gradient-echo images with 1.5×1.5×1.8mm3 resolution were simulated and downsampled separately in the left-right (downsampling-LR) and the superior-inferior (downsampling-SI) direction with different downsampling ratios using zero-padding.
Phantom: A 3D printed liver phantom filled with CuSO4-MnCl2 solutions was imaged in a water bath at 1.5T (Table 1). 3D multi-echo spoiled gradient-echo (SGRE) images with 1.3×1.3×1.3mm3 resolution were acquired in two phantom orientations and also downsampled, as in the simulation.
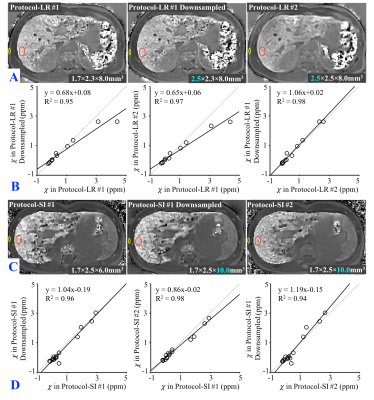
In vivo: In this IRB-approved study, 27 subjects with known or suspected liver iron overload were scanned at 1.5T after obtaining informed written consent. MRI data were retrospectively analyzed for 11 subjects imaged with two breath-held 3D SGRE-MRI with different left-right imaging resolutions, and 16 subjects imaged with two different superior-inferior resolutions (Table 1). To isolate the effect of resolution, images of higher acquired resolution (Protocol-LR #1/-SI #1) were downsampled (Protocol-LR #1/-SI #1 downsampling) to match the resolution in Protocol-LR #2/-SI#2, respectively.
Data Reconstruction and Analysis: Susceptibility maps were obtained using a liver QSM reconstruction5-6 (Figure 1).The susceptibility was measured in regions-of-interest (ROIs) drawn in different regions of the liver in simulation, phantoms and in vivo, using adjacent tissue (either subcutaneous fat or nearby water) as a susceptibility reference (yellow markers in figures). In vivo susceptibilities obtained from different protocols were compared using linear regression.
Results
In the numerical simulation (Figure 2) and the phantom (Figure 3), ringing artifacts appear at the boundaries in the susceptibility map after downsampling in both phantom-0° (top row) and phantom-90° (middle row). The low imaging resolution in different directions at a fixed downsampling ratio leads to varying biases in liver susceptibility measurements in different ROIs (bottom row). In the numerical phantom at both phantom-0°and phantom-90°, downsampling-LR (dashed lines) leads to a bigger bias in the ROI at the right of the liver (star) compared to that in downsampling-SI (solid lines) at a fixed downsampling ratio. A similar amount of bias in the ROI at the bottom (circle) and a smaller bias in the ROI at the top (triangle) in downsampling-LR were observed compared to that in downsampling-SI, respectively. In the 3D printed phantom, downsampling in LR (dashed lines) and SI (solid lines) also leads to different amounts of bias at a fixed downsampling ratio.
In Figure 4, the susceptibility map of one subject in Protocol-LR #2 (lower left-right resolution) is blurred compared to that in Protocol-LR #1 (A). The susceptibilities of all subjects measured in Protocol-LR #1 with downsampling and Protocol-LR #2 have similar values (which have the same imaging resolution but different TEs and TRs) but are both less than the estimated values in Protocol-LR#1, especially at high liver susceptibilities (B). The susceptibilities measured in all three Protocol-SI are similar (D).
Discussion and Conclusions
This study demonstrates that resolution-induced bias in liver QSM is related to the downsampling direction and is spatially-varying. Our simulation and in vivo results suggest that the liver left-right imaging resolution (typically the readout direction) may affect liver susceptibility measurements when ROI measurements are performed in the right lobe of the liver and using subcutaneous fat as a susceptibility reference. This study may guide future protocol optimization (e.g., k-space sampling approaches for liver QSM in limited scan time). Ongoing studies include comparing to susceptibility references from COSMOS7 in the 3D printed phantom or SQUID8 in the patients.Acknowledgements
The authors acknowledge support from the NIH (grants R01-DK117354, R01-DK100651, K24-DK102595, R01-DK083380, R01-DK088925, and U01-HD087216) as well as GE Healthcare who provides research support to the University of Wisconsin-Madison.The authors also gratefully acknowledge David Rutkowski and Alejandro Roldan-Alzate, Ph.D. who provided assistance with 3D liver phantom preparation.References
1. Brittenham GM, Badman DG. Noninvasive measurement of iron: report of an NIDDK workshop. Blood. 2003; 101:15-19.
2. Hernando D, et al. Quantification of liver iron with MRI: State of the art and remaining challenges. J Magn Reson Imaging. 2014; 40(5):1003-1021.
3. Zhou D, et al. Susceptibility Underestimation in a high-susceptibility phantom: dependence on imaging resolution, magnitude contrast, and other parameters. Magn Reson Med. 2017; 78:1080-1086.
4. Karsa A, et al. The effect of low resolution and coverage on the accuracy of susceptibility mapping. Magn Reson Med. 2018. DOI: 10.1002/mrm.27542.
5. Yu H, et al. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008; 60(5):1122-1134.
6. Sharma SD, et al. Quantitative susceptibility mapping in the abdomen as an imaging biomarker of hepatic iron overload. Magn Reson Med. 2015; 74(3):673-683.
7. Tian L, et al. Calculation of susceptibility through multiple orientation sampling (COSMOS): A method for conditioning the inverse problem from measured magnetic field map to susceptibility source image in MRI. Magn Reson Med. 2009; 61(1):196-204.
8. Sharma SD, et al. MRI-based quantitative susceptibility mapping (QSM) and R2* mapping of liver iron overload: Comparison with SQUID-based biomagnetic liver susceptometry. Magn Reson Med. 2017; 78(1):264-270.
Figures