4756
Reconstruction of Highly Accelerated Radial Cardiac Cine MRI using GROG based k-t ESPIRiT with TV Constraint1Electrical Engineering, COMSATS University Islamabad, Pakistan, Islamabad, Pakistan, 2Service of Radiology, Geneva University Hospitals and Faculty of Medicine, University of Geneva, Switzerland, GENEVA, Switzerland
Synopsis
Breath-hold cardiac cine MRI requires fast data acquisition with good spatio-temporal resolution. Accelerated non-Cartesian trajectories accelerate data acquisition but lead to artifacts. This work proposes a GROG-based k-t ESPIRiT approach with TV to recover the unaliased MR real-time cine images with good spatio-temporal quality. The proposed method was tested on 8 patients with single breath-hold, short-axis, real-time cardiac cine whole-heart stack with under-sampled radially acquired data using trueFISP. The efficiency of the proposed reconstruction was clinically assessed for automated segmentation, CNR & SNR and compared with the standard image reconstruction available on Siemens 3T PRISMA and 1.5T AERA scanners.
Introduction:
Cine MRI requires high spatial and temporal resolution images of the heart with breath-hold to diagnose various cardiac diseases1. Standard cine is restricted to one image per breath-hold. Radially encoded under-sampled trajectories can speed up the scan time to avoid multiple breath-holds with reduced field-of-view without wraparound artifacts and with robustness to motion. Acceleration is achieved by under-sampling, but this results in streaking artifacts2.
This work proposes the GROG-based k-t ESPIRiT with Total Variation (TV) constraint to reconstruct unaliased cardiac real-time cine MR images from highly accelerated radial data acquired using 2D multi-slice, whole heart-stack, single breath-hold, real-time cine balanced steady-state free precession (trueFISP) sequence.
Method:
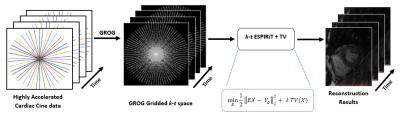
GRAPPA Operator Gridding (GROG)3 maps the acquired non-Cartesian radially encoded k-space data onto Cartesian grid by employing coil-by-coil weight sets and leaves some empty spaces in the gridded k-space3. A reconstruction method is applied to get the final solution images4.
ESPIRiT5 is an Eigen-vector-based iterative self-consistent parallel imaging reconstruction technique that uses a combination of parallel imaging and compressed sensing to get the solution image. ESPIRiT uses multi-channel receiver sensitivity profiles estimated from a calibration area (i.e. center of the acquired k-space) using Eigenvalue decomposition approach to find the solution image5.
This work proposes GROG-based k-t ESPIRiT with TV constraint for trueFISP single breath-hold real-time cardiac cine radial MR imaging to get the unaliased images with high spatio-temporal resolution. In the proposed method, initially GROG3 was used to shift the acquired radial data onto Cartesian map and then TV based k-t ESPIRiT is applied to get the final solution image. The GROG based k-t ESPIRiT with TV reconstruction is formulated as:
$$ X = minX ½||EX - Yg||22 + λTV(X) $$
where, E is the encoding operator having Fourier Transform (F) and ESPIRiT5 based receiver coils sensitivity maps (C), Yg represents the Cartesian space after GROG gridding, X is the desired solution image, TV is the total variation penalty that is used as prior knowledge and λ is the tuning factor which defines the weightage of the TV prior (empirically chosen as 0.01 in this work). In the proposed method, TV2 constraint relatively increases the spatio-temporal resolution of the resultant image. The data consistency $$$ minX ½||EX - Yg||22 $$$ is performed in Cartesian space by exploiting the empty spaces of the GROG gridded data that significantly decreases the computational complexity of the proposed scheme. Figure 1 shows a systematic depiction of the proposed method.
The proposed method is clinically tested and compared with the standard image reconstruction available on Siemens 3T PRISMA and 1.5T AERA scanners.
2D multi-slice short-axis real-time radial cine data sets were acquired from 8 patients using the trueFISP pulse sequence with 30 radial lines and 14 slices per breath-hold with TE=1.42ms, TR=43.8ms, FOV=160×160mm and resolution=1.7×1.7×8mm. The processing workstation specifications are: MATLAB R2014a, Intel (R) Core (TM) i7 4790 CPU @ 3.60GHz with 16GB RAM.
Image quality is evaluated using blood pool to myocardial Contrast-to-Noise-Ratio (CNR)6 and Myocardial and Left Ventricle (LV) Signal-to-Noise-Ratio (SNR)7 for 3 empirically chosen central slices and three cardiac phases. Automatic segmentation for function quantification is tested on all the phases of the 3 central slices. Standard t-test statistic is used for all the measured slices and all the subjects.
Results and Discussion:
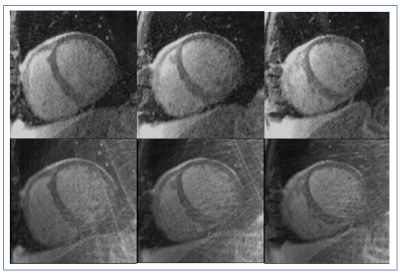
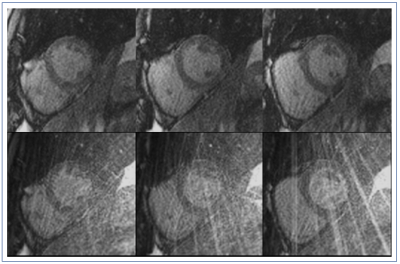
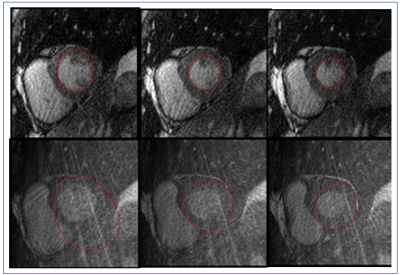
Figures 2 & 4 show 3 central slices of two different patient data sets. The proposed method provides significantly improved reconstruction results both visually with lesser artifacts and in terms of CNR and SNR. For myocardial SNR, the mean value was 3.8±1.1 for the original reconstruction and 5.5±4.3 for GROG-based k-t ESPIRiT with TV (p=0.001 showing significant improvement). For CNR, the mean value was 5.3±2.4 for the original reconstruction and 8.4±6.8 for GROG-based k-t ESPIRiT with TV (p=0.00005), showing significant improvement with GROG.
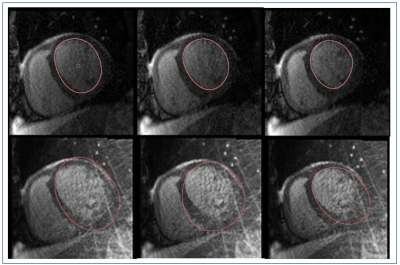
Figures 3 & 5 show that the automatic segmentation of the endocardial border (for the patients shown in Figures 2 & 4) was possible for the GROG-based k-t ESPIRiT with TV reconstructed images. Manual correction was needed for an average 10% of the images in the GROG-based k-t ESPIRiT with TV reconstructed images (6%±4% removing an outlier with 35% failed segmentations) compared to a failure rate of 62%±24% in the original reconstruction (p=0.00006 including all subjects) aiding clinical analysis workflow.
Conclusion:
The acquisition of a whole-heart short-axis stack in a single breath-hold using real-time radial cine not only reduces the exam time but avoids potential misregistration of short-axis images acquired in multiple breath-holds. The proposed method (GROG-based k-t ESPIRiT with TV) allows fast scanning and shows promising results as compared to the images provided by the scanner in terms of blood pool to myocardial CNR & SNR and allowing automatic segmentation for clinical analysis.Acknowledgements
No acknowledgement found.References
1. S.O.Schoenberg, Dietrich O, M.F.Reiser. Parallel Imaginig in Clinical MR Applications. Vol 53.; 2013.
2. Haji-Valizadeh H, Rahsepar AA, Collins JD, et al. Validation of highly accelerated real-time cardiac cine MRI with radial k-space sampling and compressed sensing in patients at 1.5T and 3T. Magn Reson Med. 2018;79(5):2745-2751.
3. Seiberlich N, Breuer F, Blaimer M, Jakob P, Griswold M. Self-calibrating GRAPPA operator gridding for radial and spiral trajectories. Magn Reson Med. 2008;59(4):930-935.
4. Aslam I, Najeeb F, Omer H. Accelerating MRI Using GROG Gridding Followed by ESPIRiT for Non-Cartesian Trajectories. Appl Magn Reson. 2018;49(1):107-124.
5. Uecker M, Lai P, Murphy MJ, et al. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med. 2014;71(3):990-1001.
6. Jeong D, Schiebler ML, Lai P. Single breath hold 3D cardiac cine MRI using kat-ARC : preliminary results at 1.5T. 2015:851-857. doi:10.1007/s10554-015-0615-0
7. Healthcare N, Ave R. Cardiac cine MR imaging: Comparison between TrueFISP and FLASH. 2001;9:2650.
Figures