4610
Eliminating susceptibility induced hyperintensities in ultra highd field T1w MPRAGE brain images1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Department of Neurology, University of Minnesota, Minneapolis, MN, United States
Synopsis
Ultra high field brain MPRAGE images are commonly affected by local susceptibility induced hyperintensities, which are pronounced in inferior frontal lobe and inferior temporal lobe. In this work, we propose a straightforward approach by applying a frequency offset of 300Hz and widening the bandwidth by 40% to the hyperbolic secant inversion pulse provided by the standard MPRAGE sequence, to eliminate this artefact without introducing additional incomplete inversion through the brain. This approach was tested across different subjects and proven to have robust performance in artefact elimination against variable local frequency offsets.
Introduction
Local hyperintensities with lack of T1 contrast are commonly seen in MPRAGE brain images acquired at ultra high field (UHF), especially in the ventral area of the frontal lobe, leading to loss of anatomical information and severe segmentation problems1. These artifacts are often close to air-tissue boundaries, stemming from local frequency offsets exceeding the inversion pulse bandwidth (BWIR) and could be mitigated by applying a frequency offset ΔfIR of 200Hz2 to the inversion pulse. However, we found this approach alone to be insufficient at 7T. Here, this artefact was largely eliminated by also increasing the inversion pulse BWIR.Methods
An MPRAGE3 sequence was customized allowing to change the duration (standard: 10240us) and center frequency ΔfIR of the hyperbolic secant inversion RF pulse (IR). Widening BWIR was obtained by shortening IR duration while increasing IR voltage to preserve adiabatic inversion. After initial optimization in 3 subjects, 9 additional subjects were scanned to evaluate the technique performance. All measurements were performed on a 7T whole body scanner (Siemens, Erlangen, Germany), equipped with a single-channel transmit and 32 channel receive head coil (Nova Medical, Wilmington, MA). ΔB0 was mapped through the brain with a gradient echo sequence. The effects of ΔfIR and BWIR were studied separately and jointly to determine optimal parameter combinations to achieve the largest spatial extent of complete inversion throughout the brain. The optimized ΔfIR was further shifted by + 60Hz or -60 Hz to evaluate the robustness of the technique against different ΔB0 distributions. Other MPRAGE parameters included: TR/TI/TE = 3100ms/1500ms/3.5ms, flip angle = 6°, FOV = 230x210x256mm3, voxel size = 0.6x0.6x0.6mm3, TA = 6:51min, GRAPPA x2. The specific absorption rate (SAR) was continuously monitored by the console. Proton density weighted (PDw) images were acquired with the same parameters but without inversion pulse (TR/TI = 2160ms/10ms, TA = 4:55min) and with a flip angle of 4°. After coregistration with SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), the ratio T1w/PDw was obtained to eliminate receive B1 field bias4.Results
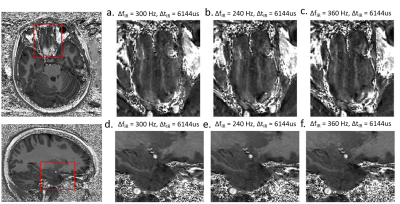
B0 maps crossing brain areas with large frequency offset are shown in Fig.1 for a typical subject. Positive frequency offsets are observed in the inferior frontal lobe and the posterior inferior temporal lobe while negative offsets are seen in the anterior inferior temporal lobe. Compared with the center of the brain, the bulk part of ΔB0 measured in the inferior frontal lobe area ranged from 240Hz to 360Hz through all subjects. When only applying either ΔfIR or increased BWIR (Fig.2 a,b; Fig.3 a.b), a large part of the incomplete inversion initially observed in the frontal lobe was effectively reduced. However, residual hyperintensities are most often still visible (Fig.2,b; Fig.3,b) where ΔB0 reaches its highest values (up to ≥300Hz). Furthermore, in some subjects, applying ΔfIR only resulted in additional hyperintensities in other brain areas subjected to local ΔB0 of opposite polarity, particularly at the infero-anterior pole of the temporal lobe (Fig.4.b). Widening BWIR efficiently addressed both artifacts (Figs.2,3 and 4). A pulse duration of 6144us together and ΔfIR=300Hz was found to be an optimal combination to achieve full inversion virtually over the whole brain. Image quality was particularly improved in the inferior frontal and temporal lobes. No difference in image quality was noticed when ΔfIR was varied from 240Hz to 360Hz (Fig.5), pointing to the robustness of the proposed technique against variations of ΔB0 through the brain between subjects. In all scan sessions SAR always stayed below the maximum allowed, which was expected given the relatively long TR between IR pulses.Discussion and conclusion
Robust elimination of susceptibility induced hyperintensities in MPRAGE 7T brain images is demonstrated by shifting IR frequency by 300Hz and shortening inversion pulse by 40%. Other methods exploiting multi-channel transmission, such as universal pulses5, can also successfully eliminate these artifacts, however, the vast majority of clinical studies rely on single channel transmit RF coils, incompatible with these approaches. Other attempts6 aiming at altering adiabatic pulse design to mitigate incomplete inversion at 7T failed to address (and sometimes aggravated) the hyperintensities and lack of T1 contrast observed in the ventral frontal lobe. We believe that our proposed method, straightforward to implement, is highly suitable for T1w image acquisition in clinical applications where losing T1 contrast in specific brain areas is a significant concern.Acknowledgements
Supporting grants: P41 EB015894 ‘Biotechnology Research Center’; P30 NS076408 ‘Institutional Center Cores for Advanced Neuroimaging’; NSF Award ID 1607835 ‘US-French Research Proposal: Hippocampal Layers: Advanced Computational Anatomy Using Very High Resolution MRI at 7 Tesla in Humans’References
1. Seiger R, Hahn A, Hummer A, et al. Voxel-based morphometry at ultra-high fields. A comparison of 7T and 3T MRI data. Neuroimage. 2015;113:207-216. doi:10.1016/j.neuroimage.2015.03.019.
2. Wiggins CJ. A Simple Method Of Improving MPRAGE Inversion Coverage at 7T. Proc Intl Soc Mag Reson Med. 2007;15:3448.
3. Mugler J. Brookeman J. Three‐dimensional magnetization‐prepared rapid gradient‐echo imaging (3D MP RAGE). Magn Reson Med. 1990;15(1):152-157
4. Van de Moortele PF et al. T1 weighted brain images at 7 Tesla unbiased for Proton Density, T2* contrast and RF coil receive B1 sensitivity with simultaneous vessel visualization. NeuroImage 2009;46:432-446;
5. Gras V et al. Universal Pulses : A new concept for calibration-free parallel transmission. Magn Reson Med 2017;77:635-6436. Wrede KH et al. Caudal image contrast inversion in MPRAGE at 7 Tesla: problem and solution. Acad Radiol. 2012;Feb;19(2):172-8.
Figures