4573
3D SPARKLING for accelerated ex vivo T2*-weighted MRI with compressed sensing1NeuroSpin, CEA Saclay, Gif-sur-Yvette, France, 2Parietal, INRIA, Saclay, France, 3ITAV, Toulouse, France, 4CNRS, Toulouse, France, 5Université de Toulouse, Toulouse, France, 6Siemens Healthineers, Saint-Denis, France
Synopsis
In the last decade, compressed sensing (CS) has been successfully used in MRI to reduce the acquisition time. Recently, we have proposed a new optimization-driven algorithm to design optimal non-Cartesian sampling patterns for CSMRI, called SPARKLING for Spreading Projection Algorithm for Rapid K-space sampLING. This method has a few advantages compared to standard trajectories such as radial lines or spirals: i) it allows to reproduce arbitrary densities while the other two are restricted to radial densities and ii) it is more robust to system imperfections. In this communication, we introduce an extension of the SPARKLING method for 3D imaging that allows to achieve an isotropic resolution of 600 μm in just 45 seconds for T2*-weighted ex vivo brain imaging at 7 Tesla.
Introduction
The design of optimal sampling trajectories for MRI in the context of compressed sensing may allow to further reduce MR scan time [1]. In [2], we have presented the non-Cartesian SPARKLING method based on the theoretical work of [3]. Using these optimized sampling patterns in 2D, high-resolution T2*-weighted in vivo brain images were rapidly acquired and presented enhanced image quality compared to spiral imaging. Here, we extend the SPARKLING method to 3D by using either a stack-of-SPARKLING strategy or fully 3D SPARKLING trajectories.Materials and Methods
- SPARKLING trajectories
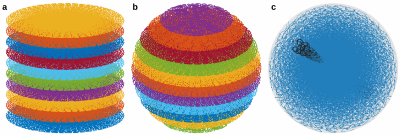
First, a regular stack-of-SPARKLING (SOS) is considered by stacking a 2D-SPARKLING trajectory along the third direction after golden-angle rotation (see Fig.1(a)).
Second, a z-variable-density SOS can be used to obtain a fully 3D variable density by changing the target density according to the plane’s altitude $$$k_z$$$. Given a discrete 3D density $$$\rho \in R^{N N N_z}$$$, a trajectory at altitude $$$k_z$$$ will be generated with the density: $$$\rho_{2D}(k_z)(:,:) =\frac{\rho(:,:,k_z)}{\int\rho(:,:,k_z)}$$$. In addition, once the number of shots in the central plane $$$n(0)$$$ is chosen, the mass of each plane can be adapted to the plane density by reducing the number of shots as $$$k_z$$$ increases: $$$n(k_z) = n(0) \frac{\int\rho(:,:,k_z)}{\int\rho(:,:,0)}$$$. Fig.1(b) illustrates such a z-variable-density stack-of-SPARKLING composed of 11 planes for an isotropic density (defined on a 3D ball).
Finally, a fully 3D SPARKLING trajectory was obtained by extending the algorithm presented in [3] to 3D. Fig.1(c) displays a fully 3D SPARKLING for 100 shots.
We compared these three SPARKLING strategies to a Cartesian iPAT4 product sequence (GRAPPA), a 3D radial trajectory [4] and Poisson Disk sampling acquired along lines in the 3rd direction as presented in [5].
- Acquisitions
3D prospective acquisitions were performed on an ex vivo baboon brain at 7 Tesla (Siemens Healthineers MR scanner, Erlangen, Germany) with a 1Tx/32Rx head coil (Nova Medical, MA, USA) and a 3D GRE sequence. The nominal gradient amplitude and slew rate were 40 mT/m and 200 T/m/s, respectively. The imaging parameters were set as follows: TR=40ms, TE=20ms, FA=15°, Tobs=15.36ms, BW=200kHz. The targeted resolution was 600μm isotropic for a field-of-view of 200x200x140mm3.
- Reconstructions
Non-Cartesian data were reconstructed by minimizing a classical CS multichannel regularized criterion balancing the trade-off between data consistency and $$$l_1$$$-based sparsity in the wavelet domain [6].
Results
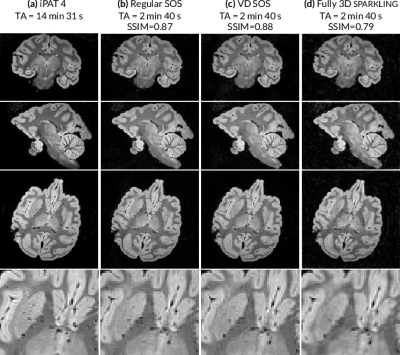
First, the different 3D SPARKLING strategies were compared for an isotropic resolution of 600μm. Regular SOS, z-variable-density SOS and fully 3D SPARKLING trajectories were acquired for an acquisition time of 2 min 40 s (4000 shots). A Cartesian iPAT4 scan (14 min 31 s) was performed and will be considered as the reference image. Results in transversal, coronal and sagittal planes are displayed in (Fig.2), where each column corresponds to a different sampling method. The image quality in the accelerated SPARKLING acquisitions is well preserved especially in the tree cerebellum. The fully 3D SPARKLING results appear slightly more blurry than the SOS techniques. Regular and z-variable-density SOS yield similar image quality as is corroborated by the SSIM scores measured on an axial slice taking the iPAT4 image as reference.
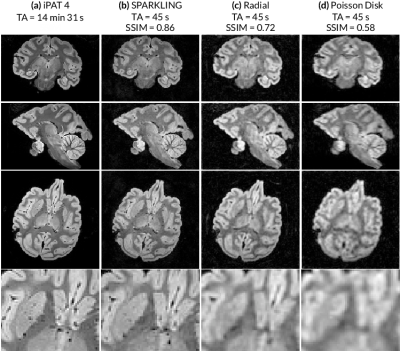
Second, 3D SPARKLING trajectories were compared to 3D radial and Poisson disk sampling strategies for a very short acquisition time of 45 seconds. Here, a z-variable-density SOS was used for SPARKLING acquisitions since it yielded better image quality among the previously tested 3D SPARKLING strategies. Moreover, a standard GRAPPA-accelerated Cartesian scan was performed for an iPAT4 as reference. Results are displayed in (Fig.3) for coronal, sagittal, axial planes and a magnified central region of the axial image, where each column displays a different strategy. Among all 45-second scans, the SPARKLING method presents the best image quality (see magnified axial slices). These visual observations are corroborated by SSIM scores, calculated for a central axial slice with the iPAT4 image as reference.
Discussion and Conclusions
In this work, we proposed to use the SPARKLING strategy to accelerate the scan time of high resolution 3D acquisitions. Among the three studied approaches of 3D SPARKLING, it was observed that the z-variable-density SOS was the most promising.
The proposed method allowed to divide the acquisition time by a factor of 20 (compared to the iPAT4 scan), while maintaining very good image quality. Moreover, we compared SPARKLING to other 3D methods such as 3D radial and the Poisson-disk-lines, for the same acquisition time of 45s. The proposed method performed significantly better than these two techniques which both appear very blurry, because of the inefficiency of their sampling lines which are too few at this acceleration rate to produce correct images. A potential straightforward application may be ultrafast susceptibility-weighted imaging [7].
Acknowledgements
This research program was supported by a 2016 DRF Impulsion grant (COSMIC, P.I.: P.C.). C.L. was also supported by the CEA international PhD program.References
[1] Michael Lustig et al. "Compressed sensing MRI". IEEE Signal Processing Magazine 25.2 (2008), pp. 72–82.
[2] Lazarus et al. "SPARKLING: Novel Non-Cartesian Sampling Schemes for Accelerated 2D Anatomical Imaging at 7T Using Compressed Sensing". 25th annual meeting of the International Society for Magnetic Resonance Imaging. 2017
[3] Boyer et al. "On the generation of sampling schemes for Magnetic Resonance Imaging". SIAM Journal on Imaging Sciences (2016); 9(4):2039–2072.
[4] Larson et al. "Anisotropic field-of-views in radial imaging". IEEE Transactions on Medical Imaging 27.1 (2008), pp. 47–57.
[5] Vasanawala et al. "Improved pediatric MR imaging with compressed sensing". Radiology 256.2 (2010), pp. 607–616.
[6] El Gueddari et al. "Self-calibrating nonlinear reconstruction algorithms for variable density sampling and parallel reception MRI". 10th IEEE Sensor Array and Multichannel Signal Processing workshop (2018).
[7] Abosch et al. "An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 Tesla". In: Neurosurgery 67.6 (2010), pp. 1745–1756.
Figures