4564
A Fast Multi-slice T1 mapping method based on SPatiotemporal ENcoding1Weizmann Institute of Science, Rehovot, Israel
Synopsis
A pulse sequence for T1 relaxation time mapping which enables high-resolution and multi-slice imaging in short acquisition
INTRODUCTION
Mapping
the spin-lattice relaxation times T1 is extensively used in MRI for a range of
applications, ranging from radiation dosimetry to evaluating the response of
tumors to therapy. T1 maps are traditionally generated using the inversion
recovery (IR) sequence, which is considered a gold standard in the field. However,
when combined with conventional multi-scan imaging techniques, IR-based
measurements result in long scanning times that become impractical for in vivo studies. In an effort to
circumvent this limitation this study presents a new methodology to achieve
multi-slice T1 maps. The new sequence combines ultrafast spatiotemporal
encoding (SPEN) MRI with Slice Shuffling (SS). SPEN is a single-shot 2D MRI pulse
sequence that can overcome image distortions arising from B0 heterogeneities,
which makes it more applicable than EPI when targeting challenging regions1,2.
SS accelerates T1 mapping by combining the IR scheme with multi-slice
excitation aspects3,4. To speed up the overall sampling procedure we
have combined slice-shuffling with SPEN, to obtain distortion-free multi-slice
T1 maps in reasonable times. The accuracy of this methodology was evaluated by
analyzing phantoms possessing different concentrations of the T1 relaxation
agent Gd-DTPA; further evaluations of the method were done with multislice T1
map acquisitions of brains and kidneys in living mice.METHOD
Figure 1a shows the new multi-slice T1 mapping pulse sequence here
proposed. This sequence combines SS and SPEN; the SS scheme is shown in further
detail in Figure 1b. It uses a nonselective adiabatic 180° RF pulse to invert all spins in the volume
of interest; this is then followed by the acquisition of multiple (N) slices,
each imaged by ultrafast SPEN while the system returns to equilibrium by
spin-lattice relaxation. The acquisition for each slice is thus performed at
progressively increasing inversion times. In order to obtain a full T1 map the
order of the N slices is altered, such that each slice is sampled at a
different inversion time. The overall experiment thus provides a number of IR
values equal to the number of slices involved. RESULTS & DISCUSSION
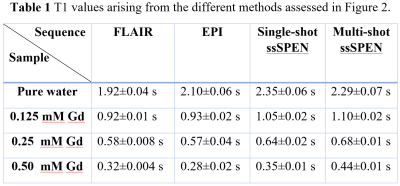
Figure 2 shows experimental results comparing T1 values arising from a phantom containing different Gd-DTPA concentrations obtained by different sequences: IR FLAIR, IR slice-shuffling multishot EPI, and IR single-/multi-shot slice-shuffling SPEN. Table 1 presents the T1 values arising from these different methods for the phantom sample; in general, the mutual agreement is very good. Figure 3 and Figure 4 show multislice T1 maps acquired by IR FLAIR, IR slice shuffling EPI and SPEN, during in vivo mouse brain and kidney studies. For the IR SS scans (EPI and SPEN) respiration gating was triggered at the beginning of each sequence; by contrast in the standard inversion recovery scan (FLAIR) the respiration gating was triggered every slice, which greatly extended the acquisition time. In order to restrict comparisons to reasonable experimental times (≈17 minutes) only 3 slices could be acquired for the IR FLAIR. This compares to the 7 slices that could be acquired for the ultrafast procedures, over total acquisition times of ≈5 minutes. Notice in this figure the improved faithfulness of the SPEN images and T1 maps over their EPI counterparts, reflecting the former’s increased robustness when targeting these challenging abdominal regions.CONCLUSIONS
By combining SPEN for accelerated image acquisitions with slice shuffling (SS) to speed up the monitoring of IR curves, a more robust method to map T1s was introduced and demonstrated. When tested on phantoms or amenable regions it provides reliable T1 maps, in agreement with standard methods; when targeting challenging abdominal regions demanding respiration triggering, it provides considerable speed improvements vs FLAIR, and fewer distortions than spin-echo EPI. Biological uses of this new capabilities are in progress.Acknowledgements
We are grateful to Mr. Koby Zibzener (Weizmann Institute) for technical assistance. Financial support came from the NIH human placenta project (R01HD086323), the Israel Science Foundation (grants 2508/17 and 965/18), the Kimmel Institute for Magnetic Resonance (Weizmann Institute) and the generosity of the Perlman Family Foundation.References
1.Tal, A. & Frydman, L. Single-scan multidimensional magnetic resonance. Prog. Nucl. Magn. Reson. Spectrosc. 57, 241–292 (2010).
2. Bao, Q., Liberman, G., Solomon, E., Lustig, M. & Fydman, L. Diffusion-weighted in vivo imaging with =100 um resolution: principles and applications to ADC mapping of pregnant mice. Proc. Intl. Soc. Mag. Res. Med. 1021 (2018).
3. Battiston, M. et al. Fast and reproducible in vivo T 1 mapping of the human cervical spinal cord. Magn. Reson. Med. 2148, 2142–2148 (2017).
4. Zhu, D. C. & Penn, R. D. Full-brain T1 mapping through inversion recovery fast spin echo imaging with time-efficient slice ordering. Magn. Reson. Med. 54, 725–731 (2005).
Figures