4563
Variable Flip Angle T1-Mapping Using Perfect In-Phase ZTEMathias Engström1, Axel Hartwig1, Cristina Cozzini2, Graeme C McKinnon3, and Florian Wiesinger2
1GE Healthcare, Stockholm, Sweden, 2GE Healthcare, Munich, Germany, 3GE Healthcare, Waukusha, WI, United States
Synopsis
This work details an extension of the Perfect In-Phase ZTE (pipZTE) method that allows for fast and efficient T1-mapping. By adding a variable flip angle scheme in combination with the Perfect In-Phase ZTE readout band-width modulation PD and T1 mapping can be achieved without interference from chemical shift artifacts.
Introduction
Recently Perfect In-Phase Zero Echo-Time (ZTE) (1) was introduced as a mean to address the chemical shift artifacts that arise when scanning ZTE over a large field of view, or when using a low readout band-width (rBW). About the same time variable flip angle ZTE was proposed (2) as a promising and efficient way of doing quantitative imaging. Using a variable flip angle scheme, in combination with a ZTE acquisition, unfortunately limits the range of possible rBWs. This due to the slab selection effects caused by prolonged RF pulse duration (required for higher flip angles) in combination with a high gradient amplitude. In this work we explore the combination of Perfect In-Phase ZTE with variable flip angle, to create quantitative T1 and proton density (PD) maps without chemical shift artifacts.Methods
A Rotating Ultra-Fast Imaging Sequence (RUFIS) (1) style Zero Echo-Time (ZTE) acquisition was modified to support both Perfect In-Phase (2), and variable flip angle (3) in one acquisition. The Perfect In-Phase rBW alternation scheme was implemented as the inner most scan loop, while the variable flip angle modification was placed as the outermost loop. This to account for the steady-state convergence time required when alternating the flip angle. For T1 and PD estimation a linearized SPGR signal model (assuming low flip angles and TR<<T1) was used (3), based on two flip angels. The chemical shift correction processing was done using a two-compartment model and single value decomposition (2), using two distinct rBWs. Reconstruction was performed on the scanner using the ORCHESTRA (ORCHESTRA, GE Healthcare, Waukesha, WI) framework and DICOM images were automatically pushed into the scanner database. A healthy volunteer was scanned on a 3.0T clinical MRI system (Signa Premier, GE Healthcare, Waukesha, WI) using a dedicated 16 channel Tx/Rx knee coil. Relevant scan parameters were FOV 160mm, resolution 1.2x1.2x1.2mm3, rBW ±31.25/25.0kHz, flip angle 4/1°, NEX 1, and the total scan duration was 3:16min.Results and Discussion
In Figure 1 ZTE data for the rBW scaling factors is shown, along with the two compartments of the Perfect In-Phase reconstruction (top row). The noticeable SNR benefit of the proposed method can be seen as a significant shift of the noise profile peak in the corresponding histograms (bottom row). In Figure 2 scan time and resolution matched ZTE is shown for a flip angle of a) 1°, and b) 4° respectively. Clear chemical shift artifacts can be seen at all tissue boundaries. A higher rBW would reduce this effect, but is not possible in this case as the duration (and consequently the BW) of the RF pulse in combination with the amplitude of the readout gradient would cause a slab selection. This would impair the image quality, contrast and consequently also the T1 and PD estimates. In Figure 2 c) the Perfect In-Phase ZTE 1° flip angle data is shown, and in Figure 2 d) the 4° flip angle data. Both present a more homogeneous signal, and the chemical shift boundary artifacts are removed. We do a acknowledge that the chemical shift correction comes with a slight resolution loss, but with the added benefit of an increased in SNR. In Figure 2 e) the PD-map is shown, and in f) the T1-map. Quantitative values in the T1-map agree with that has been reported in literature (4). We consider musculoskeletal imaging to be the primary area of application, given the relatively small FOVs required (mitigating B1+ interference and reduces gradient stress), but are also exploring the methods potential for large FOV imaging.Conclusion
We have shown that Perfect In-Phase ZTE can successfully be combined with a variable flip angle acquisition. The combination allows for chemical shift artifact free T1-, and PD-maps estimation for chemical shift artifact free. Future works includes a more comprehensive quantitative model, including B1+ correction for improved accuracy.Acknowledgements
No acknowledgement found.References
- Engström M, Cozzini C, Wiesinger F, Perfect In-Phase ZTE for improved MR Attenuation Correction. In: Proceedings of the 7th Conference on PET/MR and SPECT/MR. Elba, Italy, 2018
- Ljungberg E, Solana A-B, Wood T, Kolind S, Wiesinger F, Barker G. Silent T1-Mapping using the variable flip angle method with Zero Echo Time. In: Proceedings of the 27th Annual meeting of ISMRM. Paris, France, 2018. p. 270
- Maio DP, Lowe IJ. Ultra-fast imaging using low flip angles and fids. Magn Reson Med. 1995;34:525-529
- Han E, Gold G, Stainsby J, Wright g, Beaulieu, Brittain J. In-Vivo T1 and T2 measurements of Muskuloskeletal Tissue at 3T and 1.5T. In: Proceedings of the 11th Annual meeting of ISMRM. Toronto, Canada, 2003. p. 450
Figures
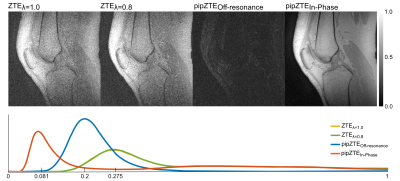
Figure1. The
top row show ZTE data with gradient scaling factors 1.0 and 0.8,
along with the two compartments of the Perfect In-Phase ZTE reconstruction
(off-resonance to the left and in-phase to the right). The corresponding
histogram for each reconstructions noise profile is shown in the bottom row.
The improved SNR property of the Perfect In-Phase ZTE is seen in the noticeable
shift of the intensity peaks.
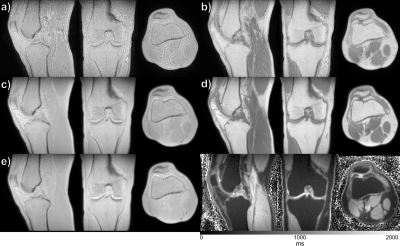
Figure 2. The
top panel show scan time and resolution matched ZTE images with clear chemical
shift artifacts (tissue delineation) for a) 1°, and b) 4° flip angle. The Perfect In-Phase ZTE equivalent
is shown in the mid panel with c) 1°, and d) 4°. e) PD- and f) T1-maps are shown in the bottom
panel, based on c) and d). Please not that c-f) are all from the same
acquisition, while a) and b) are acquired in two separate acquisitions.