4480
Navigator-less Spiral SToRM for Free breathing and Ungated Cardiac CINE MRI1Electrical Engineering, University of Iowa, Iowa city, IA, United States, 2Medicine and Biomedical Engineering, University of Virginia, Charlottesville, VA, United States
Synopsis
This study introduces an iterative kernel low-rank algorithm to recover images in a free breathing and ungated cardiac MRI dataset. The approach relies on the manifold structure of dynamic data to recover it from highly undersampled measurements. The data is acquired using variable density spiral acquisition. An iterative kernel low-rank algorithm is introduced to estimate the manifold structure of the images, or equivalently the manifold Laplacian matrix, from central k-space regions. Unlike previous manifold regularization implementations, the iterative algorithm, coupled with the non-Cartesian acquisitions, eliminates the need for dedicated navigators to estimate the manifold Laplacian, thus improving sampling efficiency.The iterative kernel low-rank algorithm facilitates the extension of manifold regularization to navigatorless spiral acquisitions, thus improving sampling efficiency. This algorithm provides improved reconstruction compared to the state of the art methods.
Background
Self-gating cardiac MRI strategies were introduced to overcome the challenges of current breath-held ECG-gated cardiac MRI acquisition techniques. Traditional self-gating strategies rely on explicit binning of data into distinct cardiac and respiratory phases, followed by image reconstruction. The recent smoothness regularization on manifold (SToRM) approach [1], which performs implicit binning of data, provides improved reconstruction of images in phases with less data. A challenge with SToRM is the need for explicit k-space navigators to estimate the manifold structure, which reduces the sampling efficiency.Methods
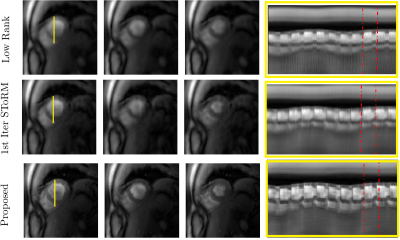
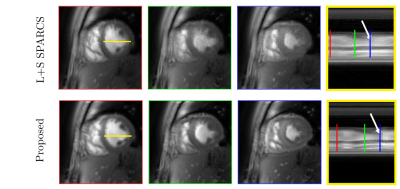
In this work, we introduce an iterative SToRM algorithm, which does not require explicit k-space navigators. We acquire the data using variable density spiral trajectories; the manifold structure is estimated from the central k-space regions with higher sampling density. Since the central k-space regions are still under sampled, we propose to use an iterative kernel low-rank method to estimate the manifold structure. The estimated manifold structure is used to recover the images from highly under sampled multi-coil spiral data in a computationally efficient manner as shown in a Figure 1. Spiral data was collected on a 3T Siemens scanner using a golden-angle rotated gradient echo spiral pulse sequence with one interleaf per excitation. Sequence parameters included: TR 7.8ms, slice thickness 8mm, resolution 1.25mm2 and total acquisition time of 16 seconds per slice. The navigator-less SToRM technique was compared against a low-rank method, 1st iteration SToRM where the manifold structure is directly estimated from multi coil SENSE reconstructed central k-space regions, and to SPARCS method [2], which is an explicit binning scheme.Results
The data was acquired from four subjects with variable breathing patterns and heart rates. Figure 2 shows the performance of the proposed method, compared to a low rank method and the first iteration of SToRM using simulated cardiac data. Figure 3 and Figure 4 show the performance of the proposed method, compared to a low rank method and the first iteration of SToRM using free breathing and ungated data, which all aim to recover the entire image time series from the measurements. Figure 5 shows a comparison between the proposed technique and SPARCS scheme, which is a self-gated strategy that aims to recover a single time series of images covering the cardiac cycle. The proposed method demonstrates superior performance as compared to the existing schemes in terms of spatial and temporal fidelity. To show the performance of our proposed method, we have compared our scheme against a low-rank method, 1st iteration SToRM (Figures 2,3,4) and SPARCS that relies on binning and motion compensation (Figure 5).Discussion
In this study, navigator-less spiral SToRM is introduced to overcome the limitation of SToRM algorithm. This approach recovers free breathing and ungated cardiac data without the requirements of explicit binning and navigator data. Results show that the less blurred images are achieved while maintaining the temporal fidelity. With the sharp endocardium and epicardium edges, we can use these images to measure the functional parameters of the heart. At the same time, with good temporal fidelity, we can also assess individual cardiac cycles which could be particularly useful in patients with irregular heart rhythms.Conclusion
Navigator less SToRM is proposed to recover the free-breathing and ungated cardiac images from variable density spiral acquisition. Both spatial images and temporal profiles show significantly reduces spatial and temporal blurring with the proposed algorithm, in comparison with the existing techniques.Acknowledgements
No acknowledgement found.References
1. Poddar, Sunrita, and Mathews Jacob. "Dynamic MRI using smoothness regularization on manifolds (SToRM)." IEEE transactions on medical imaging 35.4 (2016): 1106-1115.
2. Zhou R, Yang Y, Matthew R, Salerno M. “Cardiac and Respiratory Self-Gated Motion-Corrected Free-Breathing Spiral Cine Imaging, Proceedings of the 26th ISMRM 2018
Figures