4366
Specific quantification of 57Fe-nanoparticles by combined MRI and Mass Spectrometric Imaging1Institute of Clinical Radiology, University of Muenster, Muenster, Germany, 2Institute for Inorganic and Analytical Chemistry, University of Muenster, Muenster, Germany
Synopsis
Iron oxide nanoparticles (ION) provide high sensitivity for MRI cell tracking, but signal reductions cannot easily be separated from those originating from endogenous iron. We combine 57Fe-ION MRI with laser ablation-inductively coupled plasma-mass spectrometry for differentiation between endogenous iron (56Fe) and applied ION. We establish 57Fe-ION as contrast agent and assess their long-term fate. The technique facilitates specific and quantifiable cell tracking. ION were first internalized by phagocyting cells predominantly in liver and spleen, but in the long-term relocated to endogenous iron sources as the blood or red pulp of the spleen and, interestingly, also in the brain parenchyma.
Introduction
Iron oxide nanoparticles (ION) are common contrast agents for (pre-)clinical MRI. ION provide high sensitivity even to detect single labelled cells1,2, but signal is always influenced by endogenous iron. Hence, an exact correlation of administered ION with MRI T2/T2* quantification is not possible. We combine non-radioactive 57Fe-ION MRI with laser-ablation-mass-spectrometry (LA-ICP-MS) for differentiation between endogenous iron (56Fe) and applied ION. We assess distribution and long-term fate of administered ION, correlate ION concentration to T2-relaxivity and apply 57Fe-ION for cell tracking.Methods
Healthy C57BL/6 mice were injected with custom engineered 57Fe-ION (760µmol Fe/kg body weight, NanoPET, Berlin). For ION distribution T2 mapping of liver, spleen, kidneys and brain was performed on a 9.4T small-animal MRI after 2h, 1d, 3d, 7d, 30d and 90d (n=5 each). To study ION contribution to T2 changes with regards to applied dose, we additionally injected healthy mice with increasing ION dosage (51µmol up to 760µmol Fe/kg body weight, n=3 each). Mice were sacrificed and organs extracted for LA-ICP-MS to quantify 57Fe and the 56Fe/57Fe isotope ratio. To evaluate 57Fe-ION for cell tracking, mice were injected s.c. with 100μl of a polyacrylamide-gel (pellet) in both flanks to induce local inflammation, on one site 10µg lipopolysaccharides (LPS) was added as additional inflammatory stimulus. After 24h first MRI with T2-mapping of both pellets was performed as baseline, followed by i.v. injection of either 57Fe-ION (760µmol Fe/kg body weight, n=3) or equivalent volume of PBS as control (n=3). 24h after ION injection second MRI of the pellets with the same protocol was performed followed by sacrificing mice and pellet preparation for histology and LA-ICP-MS.Results
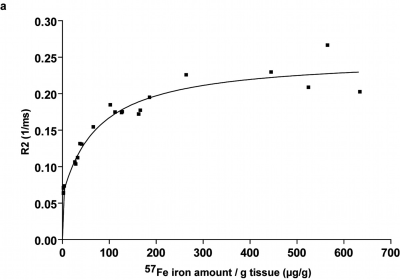
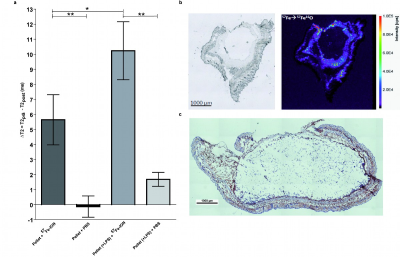
Specific detection and quantification of 57Fe-ION by ex vivo LA-ICP-MS in liver, spleen, kidneys and brain enabled for a particle distribution study after in vivo MRI (Fig.1). Most ION were first internalised by tissue macrophages in liver and spleen, where it could be well differentiated from endogeneous iron. 57Fe-signal was still found after 90d, but mainly relocated to endogenous iron stores especially in the spleen (Fig.2) and the blood (data not shown) indicating decomposition of ION. Furthermore, although low in the overall amount, increased 57Fe was detected by LA-ICP-MS within the brain parenchyma after 90d (Fig.1d), again indicating transfer of applied iron to endogenous sources. A non-linear dependence of T2-relaxivity on increasing injected iron dosage was observed in the liver, most likely resulting from ION packing, location and state during metabolic processing (Fig.3). Regarding cell tracking, 57Fe-ION MRI tracking of monocytes to local inflammation was possible and enabled in combination with LA-ICP-MS for specific detection, quantification and validation of ION based cell tracking. T2 changes were significantly higher in pellets with additional LPS than in pellets without LPS, representing a higher number of migrated labelled cells (Fig.4).Discussion
There is scarce information on ION biodistribution and long-term fate since applied iron and iron from endogenous sources overlap. Up-to-date there is no specific option for iron based imaging and validation except for the use of radioactive 59Fe-ION3, which is however associated with the drawback of radiation exposure and decay of the radioactive isotope. Here, with non-radioactive 57Fe-ION we were not only able to show the long term fate of applied ION, showing decomposition and transfer of applied iron to endogenous sources, but LA-ICP-MS was also able to resolve the local distribution of both 57Fe and 56Fe within the organ of interest in a quantitative manner. Exemplarily, LA-ICP-MS sensitively revealed increasing amounts of 57Fe in the brain parenchyma in the long-term. Furthermore, regarding cell tracking MRI and LA-ICP-MS with 57Fe-ION enabled to specifically attribute T2 signal alterations to migrated labelled cells and to distinguish from iron of other sources. Furthermore, for our approach no additional hardware is needed, as is the case for other unambiguous MRI cell tracking methods like 19F-MRI4 or hyperpolarized MRI5.Conclusion
Use of 57Fe-ION by combined MRI and LA-ICP-MS enables to study ION distribution and long-term fate, MRI iron quantification and validation of ION-based cell tracking in a specific, non-radioactive and quantitative manner. 57Fe-ION MRI thereby facilitates MRI iron quantification and validation of MRI cell tracking studies.Acknowledgements
No acknowledgement found.References
1. Masthoff M, Gran S, Zhang X, et al. Temporal window for detection of inflammatory disease using dynamic cell tracking with time-lapse MRI. Scientific reports. Jun 22 2018;8(1):9563.
2. Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magnetic resonance in medicine. Feb 2006;55(2):242-249.
3. Freund B, Tromsdorf UI, Bruns OT, et al. A simple and widely applicable method to 59Fe-radiolabel monodisperse superparamagnetic iron oxide nanoparticles for in vivo quantification studies. ACS nano. Aug 28 2012;6(8):7318-7325.
4. Schmid F, Höltke C, Parker D, Faber C. Boosting (19) F MRI-SNR efficient detection of paramagnetic contrast agents using ultrafast sequences. Magnetic resonance in medicine. Apr 2013;69(4):1056-1062.
5. Gutte H, Hansen AE, Johannesen HH, et al. The use of dynamic nuclear polarization (13)C-pyruvate MRS in cancer. American journal of nuclear medicine and molecular imaging. 2015;5(5):548-560.
Figures