4321
Ex-vivo investigation of renal metabolic heterogeneity using hyperpolarized [1-13C]pyruvate MRI: a novel renal perfusion device1Department of Clinical Medicine, the MR Research Centre, Aarhus University, Aarhus N, Denmark, 2Department of Clinical Medicine, Aarhus University, Aarhus N, Denmark, 3Department of Anesthesia and Intensive Care, Aarhus University Hospital, Aarhus N, Denmark
Synopsis
The mammalian kidney is a complex organ, maintaining the water and nutrient balance of the body. Current knowledge of the essential functions, and the interplay with metabolic processes, are mainly derived from small animal experiments (uni-papilary kidneys) using invasive methods and lacking spatial resolution. The approach presented here addresses these limitations, by introducing a new MR compatible kidney perfusion device, enabling imaging of the underlying metabolic and functional patterns associated with the multi-papillary porcine kidney, better resembeling the human physiology.
Purpose
The ability to monitor and investigate isolated organs outside the body is becoming increasingly relevant. This is due to several factors, including a need for novel transplantation applications as well as the desire to perform ever more detailed investigations into metabolism and function. Here we present the use of an in-house developed MRI compatible perfusion system, capable of investigating hemodynamic and metabolic function in ex-vivo kidney porcine models through the use of hyperpolarized and conventional MRI.Materials and methods
One kidney (125±16 g) and approximately 1.2 L
heparinized whole blood was retrieved from four fully anesthetized female pigs
(40 kg body weight), followed by the termination of the animal. The kidney was flushed with cold Ringer-acetate
(Fresenius Kabi, Bad
Homburg, GE) and cooled
down to 5 °C. The renal artery and ureter was cannulated, and the kidney was
connected to the perfusion system, see Figure 1 and 2. The perfusion system is
comprised of a
BioMedicus Medtronic Bio Console 540 centrifugal pump (Medtronic, Minneapolis,
MN, US) to maintain physiological flow at approximately 170 mL/min. The perfusate is heated to 37 °C and oxygenated using a Medos Hilite 1000 neonatal oxygenator (Xenios,
Heilbronn, GE) and a water heater/pump, see Figure 2. Temperature, flow and
pressure sensors mounted on the perfusion lines allow for continuous monitoring.
Glucose (Fresenius Kabi, Bad Homburg, GE), amino acids (Vaminolac, Fresenius Kabi, Bad Homburg, GE)
and insulin (Humulin,
Eli Lilly Demark A/S, Herlev, DK) was infused continually to keep blood gas parameters in the
physiological range1. Vasodilator (Veraloc, Orion Pharma, Copenhagen, DK) was infused to ease
the perfusion, and the produced urine was collected in a separate bag to avoid
contamination of the blood supply. Physiological and hemodynamic parameters was
monitored throughout the perfusion. The perfused kidney is placed in the bore
of a 3.0 T Signa HDx MRI scanner (GE Healthcare) equipped
with proton and carbon-13 imaging capability.
Intra-renal anatomy was assessed using T1
weighted FLAIR and T2 weighted PROPELLER imaging sequences with the
following parameters. T1 FLAIR: TR/TE/TI = 4.4 s/24.2 ms/1.6 s, flip
angle = 111°, FOV/matrix = 200×200 mm2/256×256. T2 PROPELLER: TR/TE = 7.4 s/94.1 ms, flip angle = 142°,
FOV/matrix = 200×200 mm2/256×256. Both sequences were used to acquire 15 long axis
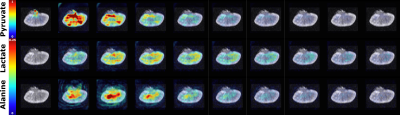
slices of 4 mm slice thickness in 2 and 4 averages respectively, see Figure 3. Metabolic
13C imaging was performed following a 9 mL injection of
hyperpolarized [1-13C]pyruvate, using a spectral spatial (SPSP)
imaging sequence with parameters: TR/TE = 0.5 s/1 ms, FOV/matrix = 120×120 mm2/128×128, one long axis slice with 40 mm slice thickness
and a 90° or 8° flip angle on lactate/bicarbonate/alanine
or pyruvate, respectively. The effective TR for pyruvate and its metabolites
was 1 and 3 seconds respectively, with three pyruvate and one
lactate/bicarbonate/alanine acquisition every 3 seconds. 1H DCE MRI was
acquired following an injection of 0.3 mL Dotarem (279.3 mg/mL) using a 3D fast
gradient echo sequence with parameters: TR/TE = 1 s/1.7 ms, flip angle = 12°,
FOV/matrix = 240×240×240 mm3/256×256.
Region of interest (ROI) analysis is performed in Matlab (MathWorks,
Natic, MA, US) using a custom segmentation method2, where each whole kidney ROI was divided into 10 equidistantly
spaced segment layers, see Figure 3.Results
Ex-vivo renal perfusion with accurate control of physiological parameters was verified with 1H MRI and [1-13C]pyruvate MRI. Our preliminary results from these investigations display differences in intra renal heterogeneity, see Figure 4. The renal cortex shows a predominant lactate production, while alanine production is mostly confined to the renal medullary region.Discussion and conclusion
Improved understanding of the role of deranged renal
metabolism in the donor graft prior to transplantation and during storage has
the potential to increase utilization of marginal organs, and thereby combat
organ shortage. This follows from the heightened potential of enhanced
therapeutic strategies, e.g. in the form of storage and pharmaceutical
interventions, with the effect of preventing graft degradation or modulating
the graft viability. With further analysis we hope to clarify the observations
presented here, and look for potential correlations between the metabolic
distribution, renal function and the outcome following transplantation.
This study demonstrates the ability to monitor ex-vivo graft metabolism and
function in a large animal model, resembling human renal physiologyAcknowledgements
No acknowledgement found.References
1. J.M. Kaths et al., "Normothermic Ex Vivo Kidney Perfusion for the Preservation of Kidney Grafts prior to Transplantation," Journal of visualized experiments : JoVE, no. 101, pp. e52909-e52909, 2015.
2. M. Pruijm et al., "Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease," Kidney International, vol. 93, no. 4, pp. 932-940, 2018.
Figures