4291
Making the Transfer: Exploiting Hyperpolarized Bicarbonate-CO2 Exchange for Increased Signal-to-Noise pH Imaging1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Bioengineering, University of California, Berkeley and University of California, San Francisco, San Francisco, CA, United States
Synopsis
Hyperpolarized (HP) [13C]bicarbonate MR imaging can map pH in vivo, but images generally suffer from low CO2 signal-to-noise ratio (SNR). However, rapid bicarbonate-CO2 chemical exchange can increase CO2 SNR via exchange-mediated polarization transfer. We exploit this phenomenon for HP [13C]bicarbonate imaging to boost CO2 SNR by 2.2-fold at pH 7.6, where CO2 SNR is lowest in the physiologic range, by acquiring and summing multiple transients. Tip angles and delays are chosen using a priori knowledge of exchange rate to increase SNR while mitigating pH error. This approach can potentially improve imaging SNR in vivo for studying extracellular acidosis in cancer.
Purpose
To increase CO2 signal-to-noise ratio (SNR) for hyperpolarized (HP) [13C]bicarbonate pH imaging by exploiting rapid bicarbonate-CO2 chemical exchangeMethods
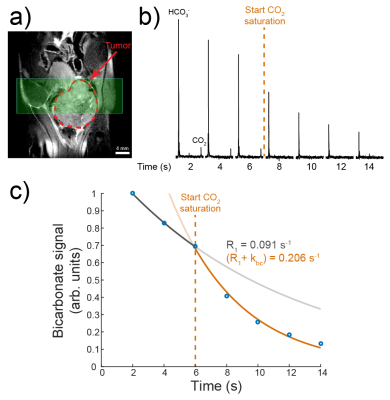
Bicarbonate-CO2 exchange measurements: Non-HP sodium [13C]bicarbonate (~20mM) was added to 100mM phosphate plus 7.55µg/mL carbonic anhydrase II (CAII) at pH 6.7. Overall bicarbonate-CO2 exchange rate was quantified via 13C selective inversion as previously described1 at 37°C on an 11.7T NMR spectrometer. Additionally, HP bicarbonate-CO2 exchange rates were measured in transgenic adenocarcinoma of the mouse prostate (TRAMP) tumors in a vertical-bore 14T MR scanner via slice-selective 13C-NMR spectroscopy plus selective CO2 saturation, as previously described2,3 (2-band Gaussian pulse4, 20° tip, 10mm slice, 2s TR, 15.5s pre-acquisition delay).
Bicarbonate-CO2 exchange simulations: HP bicarbonate+CO2 z-magnetization/signal evolution was simulated using Bloch-McConnell equations, varying pH, exchange rate, T1, tip angles, TR, and number of excitations. pH was calculated from tip angle-corrected bicarbonate/CO2 signals using a modified Henderson-Hasselbalch equation, assuming pKa = 6.172. In determining imaging parameters to maximize CO2 SNR, a |pH error| ≤ 0.05 and minimum bicarbonate:CO2 SNR ratio of 1:5 was enforced over pH 6.3-7.8.
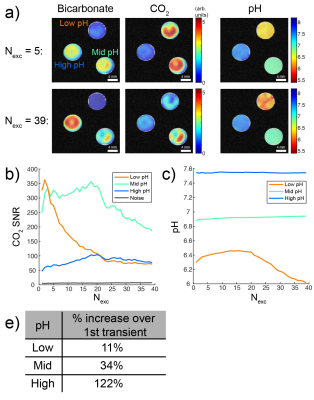
Phantom pH imaging, data processing: HP [13C]bicarbonate generated via HP [1-13C]1,2-glycerol carbonate decarboxylation4 was diluted 1:6 in three tubes containing 100mM phosphate, pH 6.4-7.6, and 7.55µg/mL CAII. A 2D echo-planar imaging (EPI) sequence (7° bicarbonate, 63° CO2, 2mm in-plane, 8mm slice, 400ms TR, 39 transients, total time 16s) with spectral-spatial excitation pulses (6.7ms pulsewidth, 300Hz spatial bandwidth, 300Hz spectral pass-band, 3624Hz spectral band separation) was then performed in a vertical-bore 14T MR scanner kept at 37°C. Using custom MATLAB scripts, raw imaging data were Fourier-transformed and pH maps calculated as described above. Regions of interest (ROIs) were drawn within each tube and in a noisy region outside the tubes, and maximum voxel CO2 SNR and average pH were plotted for each ROI as a function of the number of transients summed.
Results
The overall rate constant for CAII-catalyzed bicarbonate-CO2 exchange in phantoms was 5.51s-1 (Figure 1). Bloch-McConnell simulation predicted that by using a 9-fold higher CO2 excitation than bicarbonate, rapid exchange boosts CO2 SNR by a factor of 2.2-4.2, depending on pH, as compared with no exchange (Figure 2). This motivated further simulation to identify a sampling scheme to maximize CO2 SNR and limit pH error. A parameter scheme of [αbicarb,αCO2,TR,Nexcitations] = [7°,63°,400ms,39] was thus predicted to increase CO2 SNR by 2.9-fold over a single 90° acquisition at pH 7.8 while keeping |pH error| < 0.05 (Figure 3). This scheme was implemented in a pH phantom with HP [13C]bicarbonate using a frequency-selective 2D EPI approach. The number of summed transients to maximize CO2 SNR depended on tube pH, and average pH remained constant over time except for the low-pH tube (Figure 4), likely due to CO2 signal depletion. In order to evaluate the approach for use in vivo, bicarbonate-CO2 exchange kinetics were measured in n=3 TRAMP mice (Figure 5). Average tumor pH was 7.16±0.14, and the forward in vivo exchange rate, kbc, was 0.15±0.05s-1, agreeing with measurements in human colorectal carcinoma cell xenografts3. This corresponds with an overall exchange time constant of 1.56±0.04s-1.Discussion
We and others have previously demonstrated that exciting bicarbonate with a lower tip angle than CO2 preserves HP signal for subsequent excitations4,5. However, Bloch-McConnell simulation predicts that this unequal sampling also causes HP magnetization “shuttling” from bicarbonate to CO2 via chemical exchange over multiple acquisitions (Figure 1). This phenomenon can be exploited in a 2D EPI approach, given a priori knowledge of exchange rate, in which averaging multiple images together improves CO2 SNR, particularly for high-pH voxels, without compromising pH accuracy. Importantly, the SNR can be plotted as a function of summed transients on a per-voxel basis in order to determine where the maximum occurs, which will vary with voxel pH (Figure 4). However, pH measurement is only accurate if the injected HP bicarbonate is given sufficient time to equilibrate. Our results suggest that TRAMP prostate tumors have sufficiently rapid exchange, reaching equilibrium in ~2-2.5s (3-4 time constants). By optimizing imaging parameters for this exchange rate and an in vivo T1 of 10s2,6, the CO2 SNR can theoretically increase by 53% at pH 7.6. For TRAMP tumors, pH 7.16, the predicted SNR gain is 45% (data not shown).Conclusions
By exploiting a priori knowledge of bicarbonate-CO2 exchange, we have demonstrated significant gains in achievable CO2 SNR, particularly at high-pH values where CO2 signal is lower. Future studies will utilize these techniques for in vivo pH imaging.Acknowledgements
The authors thank all Flavell and Kurhanewicz Lab members.
Grants: R01-CA166655; R01-EB016741; R21-CA-0121429; P41-EB013598; DOD-CA-110032; DOD-PC140571P4.
References
- Dahlquist, F. W., Longmuir, K. J. & Vernet, Du, R. B. Direct Observation of Chemical Exchange by a Selective Pulse NMR Technique. J Magn Reson 406–410 (1975).
- Gallagher, F. A. et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 453, 940–943 (2008).
- Gallagher, F. A. et al. Carbonic Anhydrase Activity Monitored In Vivo by Hyperpolarized 13C-Magnetic Resonance Spectroscopy Demonstrates Its Importance for pH Regulation in Tumors. Cancer Res 75, 4109–4118 (2015).
- Korenchan, D. E. et al. Dynamic nuclear polarization of biocompatible 13C-enriched carbonates for in vivo pH imaging. Chem Commun 52, 3030–3033 (2016).
- Scholz, D. J. et al. Quantified pH imaging with hyperpolarized 13C-bicarbonate. Magn Reson Med 73, 2274–2282 (2014).
- Wilson, D. M. et al. Multi-compound polarization by DNP allows simultaneous assessment of multiple enzymatic activities in vivo. J Magn Reson 205, 141–147 (2010).
Figures