4267
Monitoring the Biodegradation of Implanted Hydrogel Cell Scaffolds using CEST MRI1Russell H. Morgan Dept. of Radiology and Radiological Science, Johns Hopkins University, Baltimore, MD, United States, 2Department of Urology, Johns Hopkins University, Baltimore, MD, United States
Synopsis
Scaffolding cells with hydrogels is a promising strategy to treat many tissue injuries and diseases. Observing hydrogel degradation is essential as it correlates to the cell release from the hydrogel. We monitored implanted hydrogel degradation using CEST MRI, with validation using two-color in vivo near-infrared imaging. An excellent correlation was found between these two methods, with gelation as the major decomposing component. The results demonstrate that CEST MRI can serve as a robust tool for monitoring hydrogel degradation in vivo.
Introduction
For cell therapy, scaffolding cells with hydrogels is a promising strategy to overcome initial cell loss and manipulate cell function post-transplantation. Matrix degradation directly influences cell release, with major effects on tissue regeneration or repair.1 Monitoring such degradation is essential for developing scaffolded cell-based therapies. We applied chemical exchange saturation transfer (CEST) MRI to monitor hydrogel degradation in vivo in a label-free fashion, using a commonly used hyaluronic (HA)-gelatin composite hydrogel. The abundant exchangeable protons in the hydrogel make it possible to visualize the matrix using CEST MRI without the need of exogenous labels.Methods
A covalently cross-linked hydrogel composing of 20 mg/mL thiol-modified gelatin (Gel-SH), 20 mg/mL thiol-modified HA (HA-SH), and 20 mg/mL polyethylene (glycol) diacrylate (PEGDA) (crosslinker) was prepared (Fig. 1a). In vitro CEST MRI was carried out at 37 °C. Mouse glial restricted progenitor cells (mGRPs) were scaffolded within the hydrogels to assess cell survival and proliferation. For in vivo visualization of degradation, 3 μL hydrogel was injected into mouse striatum. CEST MRI was performed using a horizontal bore 11.7 T Bruker scanner up until 42 days. To validate the in vivo CEST MRI findings and identify the main decomposing component in the hydrogel, gelatin and HA were labeled with green and red near-infrared (NIR) dyes, respectively. The labeled hydrogel was then visualized using a LI-COR optical in vivo imaging system. Additionally, mGRPs transduced with a lentiviral vector carrying firefly luciferase (pLenti4-CMV-Luc) were scaffolded within hydrogels and injected into mouse brain. The survival of transplanted cells with and without scaffolding was then monitored by bioluminescence imaging (BLI).Results and Discussion
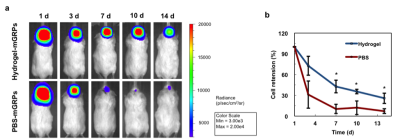
In vitro CEST MRI demonstrated a stable peak at 3.6 ppm for saturation field strengths (B1) from 1.2 to 7.2 μT. A value of 3.6 μT exhibited a comparable CEST signal with higher B1 values (Fig. 1b), but a less broad spectrum, and was therefore used for all further experiments. When we examined the three individual components of the hydrogel, we found that gelatin is the major contributor to the CEST signal at 3.6 ppm (Fig. 1c), in agreement with earlier studies.2 An increase of the gelation proportion increased the CEST signal (Fig. 1d-e). When the hydrogel was injected into the brain of mice, it could be clearly distinguished from the surrounding brain tissue (Fig. 1f-g). In vitro cell studies showed that mGRPs survived for with more than 4 months with >60% viability in all four tested hydrogels (Fig. 2a-b). Cells continued to proliferate for at least 15 days in hydrogels, with the proliferation rate correlating to the hydrogel stiffness (Fig. 2c). A 4:1 hydrogel ratio showed better cell proliferation relative to 2D cell cultures (Fig. 2d). When the 4:1 hydrogel was injected into the brain of mice, a decrease in CEST MRI signal was observed over time (Fig. 3a-b). The NIR signal of gelatin decreased gradually over 42 days, while the HA signal remained relatively stable (Fig. 3c). This suggests that gelatin, the main source of CEST signal at 3.6 ppm, is the major decomposing component in the hydrogel. An excellent correlation was found between the decay of CEST signal and gelatin NIR fluorescence signal (R2=0.94, Fig. 3d). When scaffolded mGRPs were implanted into the brain striatum, the cells showed a prolonged in vivo survival and restricted localization at the injection/target sites relative to un-scaffolded (naked) mGRPs (Fig. 4a-b).Conclusions
Hydrogel degradation can be monitored using CEST MRI. Gelatin was found to be the major contributor of CEST contrast and also the main degradation component in the hydrogel. Scaffolding GRPs in hydrogels can confer higher graft stability with minimal cell loss, leading to prolonged cell survival. Therefore, this approach may be used further to develop hydrogels with optimal biodegradation properties for delivering cells.Acknowledgements
No acknowledgement found.References
1. Madl CM, Lesavage BL, Dewi RE et al.: Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodeling. Nature Materials. 2017;16:233-1242.
2. Liang Y, Bar-Shir A, Song X, Gilad AA, Walczak P, Bulte JWM: Label-free imaging of gelatin-containing hydrogel scaffolds. Biomaterials. 2016;42:144-150.
Figures

Figure 1. In vitro and in vivo CEST MRI. (a) Scheme of hydrogel preparation. (b) Dependence of hydrogel MTRasym on different saturation powers. (c) MTRasym values of the three individual hydrogel components for a saturation power of 3.6 μT. (d) MTRasym values of hydrogels with different Gel-SH to HA-SH ratios. (e) Quantitative MTRasym values and CEST maps at 3.6 ppm. (f) Hydrogels injected into striatum of mouse brain are distinguishable from surrounding host tissue (arrow indicates needle track). (g) MTRasym measurements in the implanted scaffold region-of-interest demonstrate a stable peak at 3.6 ppm.