4219
Development of a human heart sized perfusion system for metabolic imaging studies using hyperpolarized [1-13C]pyruvate MRI1Department of Clinical Medicine, the MR Research Centre, Aarhus University, Aarhus N, Denmark, 2Department of Anesthesia and Intensive Care, Aarhus University Hospital, Aarhus N, Denmark, 3Department of Cardiothoracic Surgery, Aarhus University Hospital, Aarhus N, Denmark
Synopsis
In order to combat the increasing worldwide organ shortage, increased understanding of the role of deranged metabolism in the donor organ is vital. This is especially true in the case of the heart, where the ability to assess cardiac metabolism during storage pending transplantation, has the potential to utilize marginal organs and elevate the graft viability. Here we present the development of a MR compatible perfusion system used in combination with hyperpolarized [1-13C]pyruvate and conventional MRI. This enables detailed investigations into ex-vivo cardiac metabolism and function in a large animal model, resembling the case in humans.
Purpose
An increasing number of patients worldwide are awaiting organ transplantation, which is directly leading to an increased organ shortage1. This warrants new strategies to improve the outcome and donor pool size. Transplantation using organs donated after circulatory death often increase the risk of delayed functional restoration or even lost function of the graft. Especially hearts are often discharged due to a restricted transplantation safety level2. Highly sensitive measures for estimation of organ viability is therefore needed to improve the handling procedures of the organs from extraction to insertion in the recipient. This study introduces a novel MRI compatible porcine organ perfusion device for ultra-sensitive quantification, using hyperpolarized and conventional MRI, of the most important metabolic pathways to monitor graft viability of the heart.Materials and methods
A MRI compatible perfusion chamber holds
the organ which is supplied with oxygen and nutrients using approximately 1.2 L
heparinized whole blood. A BioMedicus
Medtronic Bio Console 540 centrifugal pump (Medtronic, Minneapolis, MN, US) maintains
physiological perfusion pressure at 85 ± 5 mmHg. The perfusate is heated and
oxygenated using a Medos Hilite 1000 neonatal oxygenator (Xenios, Heilbronn, GE)
and a water heater/pump, see Figure 1. Temperature, flow and pressure sensors mounted
on the perfusion lines allow for continuous monitoring. A liquid
filled latex balloon inserted in the left ventricle allows the heart work
and enables monitoring of the heart rate via a pressure transducer, which can
be used for cardiac gating during the acquisition of MRI data.
Prior to the ex-vivo perfusion MRI experiment,
the heart and perfusate is retrieved from fully anesthetized female pigs (40 kg
body weight). The heart is arrested using a cold 1:4 mixture of Harefield cardiolpegia
solution (Terumo BCT Ltd., Larne, UK) and Ringer Lactate (Fresenius Kabi, Bad Homburg, GE) just prior to the removal of the organ, and subsequently kept cool at 5° C until the start of perfusion. A
cannula is applied to the aorta3 and the balloon is inserted into
the left ventricle. The heart is perfused at 38° C in reverse, shutting the aortic valve and forcing the perfusate into the coronary vessels,
see Figure 2. Glucose (Fresenius Kabi,
Bad Homburg, GE) and insulin (Humulin,
Eli Lilly Demark A/S, Herlev, DK) is infused continually. The perfusion chamber
was placed in a 3.0 T Signa HDx MRI scanner (GE Healthcare) equipped with proton and carbon-13 imaging
capability, see Figure 2.
Cardiac anatomy was assessed using T1 weighted
FLAIR and T2 weighted PROPELLER imaging sequences with the following
parameters. T1 FLAIR: TR/TE/TI = 4.4 s/24.2 ms/1.6 s, flip angle = 111°,
FOV/matrix = 200×200 mm2/256×256. T2 PROPELLER: TR/TE = 7.4 s/94.1 ms, flip angle = 142°, FOV/matrix = 200×200 mm2/256×256. Both
sequences were used to acquire 15 slices of 4 mm slice thickness in 2 and 4 averages
respectively, see Figure 3. CINE imaging was performed to visualize
cardiac function: TR/TE = 5.45 ms/2.59 ms, flip angle = 55°, FOV/matrix = 120×120 mm2/512×512 in 30
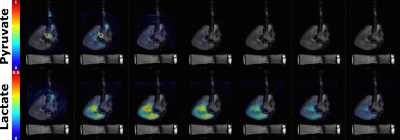
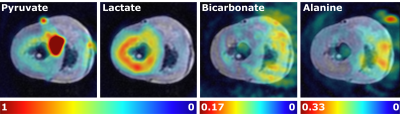
images throughout the cardiac cycle. 13C imaging was
performed, following a 9 mL injection of hyperpolarized [1-13C]pyruvate, using a spectral spatial (SPSP) imaging sequence with parameters: TR/TE = 0.5 s/1 ms, FOV/matrix = 120×120 mm2/128×128, one long
axis slice with 40 mm slice thickness and a 90° or 8° flip angle on lactate/bicarbonate/alanine or pyruvate, respectively.
The effective TR for pyruvate and its metabolites was 1 and 3 seconds
respectively. Imaging was performed in either long or short axis
orientation.Results
Three porcine hearts (238 ± 43 g) were investigated ex-vivo, using the developed perfusion system. In cases where the heart did not defibrillate spontaneously upon reperfusion, electrical defibrillation was performed. The heart rate was in the range 68-75 BPM. Ex-vivo cardiac perfusion with accurate control of physiological parameters was verified with 1H MRI and [1-13C]pyruvate MRI. Preliminary results from the hyperpolarized experiments are shown in Figure 3 and 4. A predominant lactate production was observed in all the pilot experiments.Discussion and conclusion
Increased understanding of the role of deranged cardiac
metabolism in donor grafts during storage pending transplantation has the
potential to increase utilization of marginal organs, thereby combatting organ
shortage. This is due to the heightened potential of enhanced therapeutic
strategies in the form of novel storage solutions and pharmaceutical
interventions, with the effect of preventing the degradation of the and
modulation of the graft viability. With further work and analysis, we hope to
investigate the metabolism of the ex-vivo porcine heart as well as the effect
of pre-conditioning strategies on graft viability. The preliminary results shown
here demonstrates the ability to monitor ex-vivo cardiac metabolism and
function in a large animal model, resembling the case in humans.Acknowledgements
No acknowledgement found.References
1. R. Girlanda, "Deceased organ donation for transplantation: Challenges and opportunities," World journal of transplantation, vol. 6, no. 3, pp. 451-459, 2016.
2. H.T. Tevaearai Stahel et al., "Hearts Not Dead after Circulatory Death," (in English), Frontiers in Surgery, Opinion vol. 2, no. 46, 2015.
3. M.A. Schechter et al., "An Isolated Working Heart System for Large Animal Models," JoVE, no. 88, p. e51671, 2014.
Figures