4124
Design of a 3D Printed Patient-Specific Dual Compartment Breast Phantom1Bernard and Irene Schwartz Center for Biomedical Imaging and CAI2R, NYU Langone Health, New York, NY, United States
Synopsis
Presented here is the design of a 3D printed anthropomorphic breast phantom that includes the key breast tissue compartments which can be filled with desired tissue-mimicking fluids. The breast phantom can be used for validating MRI, coil development, safety evaluation and pulse sequence evaluation.
Introduction
A well-known means for validating MRI acquisition and analysis techniques is through the use of physical phantoms, i.e. objects of known geometries and/or composition. A number of MRI phantoms are commercially available; however, these phantoms tend to be costly and feature simplified geometries [1-3]. Many applications may require customizable and anatomically realistic configurations, which may be achievable using three-dimensional (3D) printing technologies [4]. The purpose of this study was to assess the feasibility of manufacturing an anatomically accurate 3D printed anthropomorphic breast phantom that includes the key breast tissue compartments.Methods:
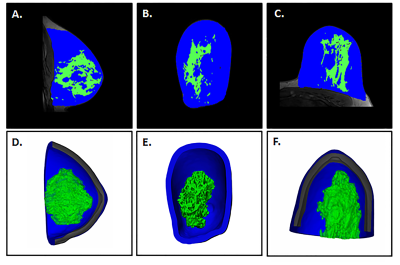
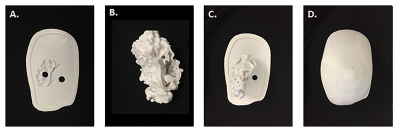
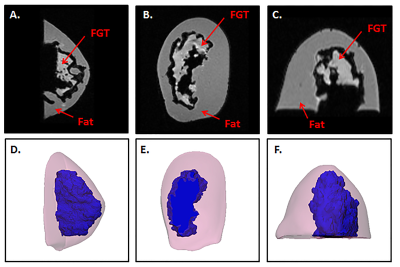
Fat and fibroglandular tissue (FGT) were segmented from MRI data and 3D volumes were created and hollowed with a 7 mm external wall thickness for the fat and 3 mm thickness for the FGT (Mimics 21.0 and 3-matic 13, Materialise, Leuven, Belgium), (Figure 1). A base was designed with an engraving to align the FGT with the other compartments that were also fitted with individual fill ports. Each part was 3D printed with a 0.178 mm layer thickness (Fortus 360mc, Stratasys, Eden Prairie, MN) and sealed (Figure 2); and the components were assembled. To mimic the dielectric properties of human breast tissue, the fatty compartment was filled with peanut oil (σ =0.1 S/m, εr =7.4) and the FGT compartment was filled with a polyvinylpyrrolidone-based phantom material (σ =0.3 S/m, εr =45) [5,6]. MR images of the 3D printed phantom were acquired on a 7T system (Magnetom, Siemens, Erlangen, Germany) using a Siemens 7.0T Tim Head Coil (Invivo Corp., Pewaukee, WI). A 3D volumetric sequence with a spatial resolution of 1.1 mm x 0.7 mm x 0.7 mm was used, with the following imaging parameters: FA =10˚, TR = 7.61 ms, TE 2.00 ms, Averages =7. The fatty and FGT compartments visualized in the phantom model were segmented in Mimics 21.0, the segmented volumes were compared to those obtained from the original MRI data, and the DICE similarity coefficient (DSC) was calculated.Results
The 3D printed phantom was successfully configured and no leaks were observed. Figure 3 shows MR images and corresponding 3D reconstructions of the 3D printed breast phantom model. The 3D phantom demonstrated a good visual match to the original 3D model. The volume of the segmented fat was 169,083 mm3 and 151,195 mm3 on the original MRI images and 3D printed phantom images respectively (DSC = 0.94) and the FGT volumes were 45,290 mm3 and 56,514 mm3 respectively (DSC = 0.89).Discussion and Conclusion
3D printing offers novel and versatile opportunities for generating realistic distribution of human tissue. We have demonstrated the feasibility of constructing an accurate breast phantom that mimics the complex FGT and fat distribution in an individual patient. The phantom allows each compartment to be filled with desired tissue-mimicking fluids. In general, MRI phantoms may be used for routine quality assessment tests that are necessary to allow for quantitative imaging, e.g. diffusion weighted imaging, MR spectroscopy, especially in multicenter studies across different vendors and magnetic field strengths. The anthropomorphic 3D printed breast phantom presented here can be used for validating MRI, coil development, safety assessment, and pulse sequence evaluation. As new 3D printing technologies and materials emerge, we expect to be able to create highly accurate 3D printed anthropomorphic phantoms with even more complex geometries.Acknowledgements
This work was supported by the Center for Advanced Imaging Innovation and Research (www.cai2r.net), a NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).References
1. Russek SE, Boss M, Jackson EF, Jennings DL, Evelhoch JL, Gunter JL, Sorensen G. Characterization of NIST/ISMRM MRI System Phantom. Proceedings of the ISMRM, 2012 Melbourne, Australia.
2. Bosca R, Ashton E, Zahlmann G, Jackson E, editors. RSNA Quantitative Imaging Biomarker Alliance (QIBA) DCE-MRI phantom: goal, design, and initial results. In Proceedings of the 98th Scientific Assembly and Annual Meeting of RSNA, Chicago, Illinois, USA, 2012.
3. Chen CC, Wan YL, Wai YY, Liu HL. Quality assurance of clinical MRI scanners using ACR MRI phantom: preliminary results. J Digit Imaging. 17(4):279–84, 2004.
4. Wood S, Krishnamurthy N, Santini T, Raval S, Farhat N, Holmes JA, Ibrahim TS. Design and fabrication of a realistic anthropomorphic heterogeneous head phantom for MR purposes. PLOS ONE 12(8): 20183168, 2017.
5. Ianniello C, de Zwart JA, Duan Q, Deniz C, Alon L, Lee JS, Lattanzi A, Brown R. Synthesized tissue-equivalent dielectric phantoms using salt and polyvinylpyrrolidone solutions. Magn Reson Med 80:413-419, 2018.
6. https://amri.ninds.nih.gov/cgi-bin/phantomrecipe
Figures