4112
Diffusion-weighted imaging with apparent diffusion coefficient (ADC) mapping in non-mass enhancing breast tumors: influence of region-of-interest placement in diagnostic accuracy and intra-/inter-reader agreementDaly Avendano1,2, Maria Adele Marino1,3, Doris Leithner4, Blanca Bernard-Davila4, Maxine Jochelson4, Elizabeth Morris4, Pascal Baltzer5, Thomas Helbich5, and Katja Pinker4,5
1Breast imaging service, Memorial Sloan Kettering Cancer Center, New York, NY, NY, United States, 2Breast imaging, Department of Breast Imaging, Breast Cancer Center TecSalud, ITESM Monterrey, Nuevo Leon, Mexico, Monterrey, Mexico, 3Department of Biomedical Sciences and Morphologic and Functional Imaging, University of Messina, Messina, Italy, Department of Biomedical Sciences and Morphologic and Functional Imaging, University of Messina, Messina, Italy, Messina, Italy, 4Breast imaging service, Memorial Sloan Kettering Cancer Center, New york, NY, United States, 5Department of Biomedical Imaging and Image-guided Therapy, Division of Molecular and Gender Imaging, Medical University Vienna, Vienna, Austria, Department of Biomedical Imaging and Image-guided Therapy, Division of Molecular and Gender Imaging, Medical University Vienna, Vienna, Austria, Viena, Austria
Synopsis
Non-mass enhancing (NME) breast tumors are a diagnostic challenge and limited data exists for characterization with DWI with ADC mapping. We aim to investigate the influence of ROI placement and different ADC metrics on diagnostic accuracy of DWI and to assess intra and inter-reader agreement of ADC measurements with DWI in NME breast lesions. Two readers independently assessed DWI using three different ROI´s approaches for ADC measurements. Data indicate that diagnostic accuracy of DWI with ADC mapping is limited in lesions presenting as NME on DCE-MRI with only moderate inter-reader agreement
Introduction
Non-mass enhancing (NME) lesions are a diagnostic challenge and one of the most frequent causes of false-positives findings with DCE-MRI [1] with a wide spectrum of both benign and malignant diseases associated [2]. Diffusion-weighted imaging (DWI) is a non-contrast MRI parameter, which provides a strong surrogate marker for tissue microstructure, membrane integrity, and cell density, and can be quantified by calculation of the apparent diffusion coefficient (ADC) [3]. Decreased ADC values indicate a diffusion hindrance, indicative of malignancy [4–6]. Sensitivities of DWI with ADC mapping of up to 96% for breast cancer detection and specificities of up to 100% for breast tumor characterization have been reported [7–11]. Limited data for NME characterization with DWI, suggesting is less sensitive and specific in these diagnostically challenging lesions. It has been shown that 2D ROI ADC measurement approach in the part of the enhancing tumor with the visually assessed lowest ADC is the most practical [12–14], it remains unclear the best measurement approach in NME. The aim of the study was to investigate the influence of region-of-interest (ROI) placement and different ADC metrics on diagnostic accuracy of DWI and to assess intra and inter-reader agreement of ADC measurements with DWI in NME breast tumorsMethods
In this IRB-approved single-institution study and retrospective data analysis 95 patients, with multiparametric MRI (DCE/DWI) and diagnosed with NME breast lesion (BI-RADS 4/5) were included. 29 had to be excluded due to; suboptimal quality n=5, non-visibility n=24 of the lesion on DWI. Two readers independently assessed DWI and DCE in two separate randomized readings. For DWI readers recorded ADC mean values using a) Whole tumor ROI, b) Darkest part tumor 10 mm ROI and c) point tumor 3 mm ROI, (Fig 1). NME were classified as a benign (>1.3 x10-3 mm2/s) or malignant (1.3 x 10-3 mm2/s). Histopathology as standard of reference. ROC curves were plotted and the area under the curve (AUC) was determined. Concordance correlation coefficient (CCC) was performed for agreement on continuous measures (MRI features) obtained by the two independent readersResults
66 patients (mean ages 51.8 ±10.8 yo, range 26-76 yo) were included for analysis with a mean size lesion of 40±25 mm, range 5 mm-98 mm): 39 malignant (59%) and 27 benign (41%). The best diagnostic performance for DWI for both readers and readings were achieved with Dptu mean with AUC range 0.512 and 0.709 (Figure 2). CCC varied among the different ADC metrics was fair-to-moderate. The best results for CCC were achieved for Dptu max ADC (0.420) in the second reading (Figure 3). Similar results were obtained for the intra-reader agreement with the best CCC for Wtu mean with 0.435 for R1 and 0.412 for R2 (figure 4)Discussion
The recent concerns about the safety of gadolinium contrast agents, recommendations state “gadolinium based contrast agents should only be administered if the information so provided is necessary, and specifically expected to increase the confidence in correct disease diagnosis or assessment thereof, or disease exclusion” [15]. Several unenhanced MR imaging parameters have been explored in this context. Advances in imaging techniques and hardware, as better gradient systems and multi-channel coils have overcome some limitations such as susceptibility and respiratory motion artifacts, establishing DWI as an essential part of oncologic imaging protocols. DWI with ADC mapping can be easily implemented in a routine breast MRI protocol without significantly increasing scan time [19, 20], has emerged as the most robust and reliable adjunct to DCE-MR with sensitivities of up to 96% and specificities of up to 100% [4-6]. The majority of data available is for mass enhancing tumors and there are concerns that DWI is limited in small masses and NME lesions [9]. Our results confirm that diagnostic accuracy of DWI with ADC mapping is limited in lesions presenting as NME on DCE-MRI with only moderate inter-reader agreement. Whereas Wtu ADC mean measurement is the most repeatable, 2DROI ADC measurements is the most accurate for breast cancer diagnosis. It has to be noted, in this study 24 (27%) patients with NME on DCE-MRI the lesion (12 benign/ 12 malignant) was not discernible on DWI. A limitation of our study is the small number of cases, yet to our knowledge is the largest series of that investigated MpMRI of the breast for NME lesions. Our findings need to be verified in further larger-scale studiesConclusions
Diagnostic accuracy of DWI with ADC mapping is limited in breast tumors presenting as NME. Best results are achieved using 2D ROI measurements approach and ADC mean. A substantial number of lesions not visible on DWI alone and therefore DCE-MRI is still indispensable for detection and characterization of breast cancers presenting NME lesions.Acknowledgements
No acknowledgement found.References
[1] Baltzer PAT, Kaiser WA, Dietzel M (2015) Lesion type and reader experience affect the diagnostic accuracy of breast MRI: a multiple reader ROC study. Eur J Radiol 84:86–91 . doi: 10.1016/j.ejrad.2014.10.023 [2] Dorrius MD, Dijkstra H, Oudkerk M, Sijens PE (2014) Effect of b value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: a systematic review and meta-analysis. Eur Radiol 24:2835–2847 . doi: 10.1007/s00330-014-3338-z [3] Baltzer P a. T, Renz DM, Herrmann K-H, et al (2009) Diffusion-weighted imaging (DWI) in MR mammography (MRM): clinical comparison of echo planar imaging (EPI) and half-Fourier single-shot turbo spin echo (HASTE) diffusion techniques. Eur Radiol 19:1612–1620 . doi: 10.1007/s00330-009-1326-5 [4] Marini C, Iacconi C, Giannelli M, et al (2007) Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur Radiol 17:2646–2655 . doi: 10.1007/s00330-007-0621-2 [5] Guo Y, Cai Y-Q, Cai Z-L, et al (2002) Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging JMRI 16:172–178 . doi: 10.1002/jmri.10140 [6] Yankeelov TE, Luci JJ, Lepage M, et al (2005) Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: a reference region model. Magn Reson Imaging 23:519–529 . doi: 10.1016/j.mri.2005.02.013 [7] Bogner W, Pinker-Domenig K, Bickel H, et al (2012) Readout-segmented echo-planar imaging improves the diagnostic performance of diffusion-weighted MR breast examinations at 3.0 T. Radiology 263:64–76 . doi: 10.1148/radiol.12111494 [8] Chen X, Li W, Zhang Y, et al (2010) Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer 10:693 . doi: 10.1186/1471-2407-10-693 [9] An YY, Kim SH, Kang BJ (2017) Differentiation of malignant and benign breast lesions: Added value of the qualitative analysis of breast lesions on diffusion-weighted imaging (DWI) using readout-segmented echo-planar imaging at 3.0 T. PloS One 12:e0174681 . doi: 10.1371/journal.pone.0174681 [10] Filli L, Ghafoor S, Kenkel D, et al (2016) Simultaneous multi-slice readout-segmented echo planar imaging for accelerated diffusion-weighted imaging of the breast. Eur J Radiol 85:274–278 . doi: 10.1016/j.ejrad.2015.10.009 [11] Clauser P, Marcon M, Maieron M, et al (2015) Is there a systematic bias of apparent diffusion coefficient (ADC) measurements of the breast if measured on different workstations? An inter- and intra-reader agreement study. Eur Radiol. doi: 10.1007/s00330-015-4051-2 [12] Arponen O, Arponent O, Sudah M, et al (2015) Diffusion-Weighted Imaging in 3.0 Tesla Breast MRI: Diagnostic Performance and Tumor Characterization Using Small Subregions vs. Whole Tumor Regions of Interest. PloS One 10:e0138702 . doi: 10.1371/journal.pone.0138702 [13] Lambregts DMJ, Beets GL, Maas M, et al (2011) Tumour ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol 21:2567–2574 . doi: 10.1007/s00330-011-2220-5 [14] Bickel H, Pinker K, Polanec S, et al (2017) Diffusion-weighted imaging of breast lesions: Region-of-interest placement and different ADC parameters influence apparent diffusion coefficient values. Eur Radiol 27:1883–1892 . doi: 10.1007/s00330-016-4564-3 [15] Gulani V, Calamante F, Shellock FG, et al (2017) Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 16:564–570 . doi: 10.1016/S1474-4422(17)30158-8 [16] Marino MA, Helbich T, Baltzer P, Pinker-Domenig K (2018) Multiparametric MRI of the breast: A review. J Magn Reson Imaging JMRI 47:301–315 . doi: 10.1002/jmri.25790 [17] Pinker K, Marino MA, Dr Meyer-Baese A, Helbich TH (2016) [Multiparametric and molecular imaging of breast tumors with MRI and PET/MRI]. Radiol 56:612–621 . doi: 10.1007/s00117-016-0129-3 [18] Pinker K, Moy L, Sutton EJ, et al (2018) Diffusion-Weighted Imaging With Apparent Diffusion Coefficient Mapping for Breast Cancer Detection as a Stand-Alone Parameter: Comparison With Dynamic Contrast-Enhanced and Multiparametric Magnetic Resonance Imaging. Invest Radiol. doi: 10.1097/RLI.0000000000000465 [19] Bogner W, Pinker-Domenig K, Bickel H, et al (2012) Readout-segmented echo-planar imaging improves the diagnostic performance of diffusion-weighted MR breast examinations at 3.0 T. Radiology 263:64–76 . doi: 10.1148/radiol.12111494 [20] Chen X, Li W, Zhang Y, et al (2010) Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer 10:693 . doi: 10.1186/1471-2407-10-693Figures

Figure 1. Magnetic resonance of the breast with apparent
diffusion coefficient map images (a,b,c), axial view. The images show a
hypointense area of restricted diffusion in the central part of the right breast.
Examples of the three methods used to measure the apparent diffusion
coefficient values: a) whole tumor delineation, b) darkest part of the tumor
and c) point tumor delineation. The
three ROIs show low ADC values <1.3x10-3mm2/s
indicating highly suspicious of malignancy. Final histopathological diagnosis
yielded invasive ductal carcinoma, G3
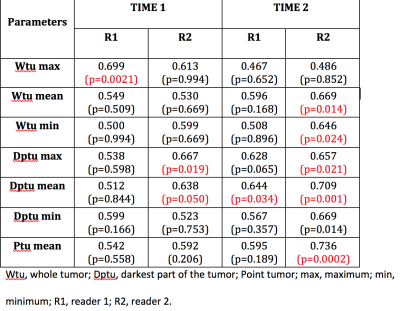
Figure 2. Area
under the curve for both readers at the two time points with the three methods
of segmentation
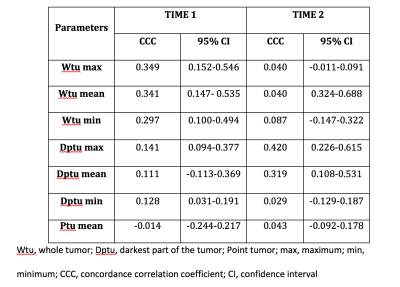
Figure 3. Inter-reader agreement, concordance correlation
coefficient and the 95% confidence interval for all measured MRI parameters in
Time 1 and Time 2
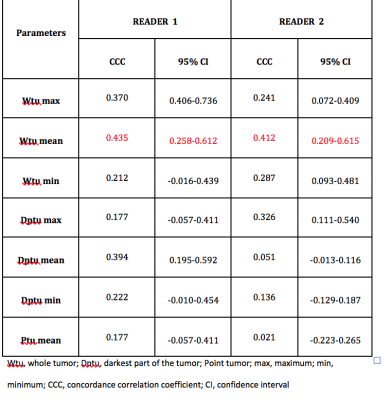
Figure 4. Intra-reader agreement and 95% coefficient interval
for Reader 1 and Reader 2 in two times readings