4051
DCE-MRI texture analysis in differentiating adenocarcinoma and squamous cell cancer of cervix1Radiology, Cental Hospital of Wuhan,Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2Radiology, Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 3Philips Healthcare, Shanghai, China
Synopsis
Adenocarcinoma comprises 25% of cervical cancers
and has a bad prognosis and poor outcome of radiotherapy and chemical treatment
in the advanced stage. Here, we report a radiomics method with multi-class texture features from
semi-quantitative DCE-MRI maps to distinguish adenocarcinoma from squamous cell
cancer. Multivariate
models were trained on the training cohort and their performance was evaluated
on the 5-fold cross-validation cohort using the area under ROC curve (AUC),
accuracy, specificity and sensitivity. Our results showed the
mean sensitivity, specificity, PPV, NPV and AUC were 0.96, 0.889, 0.967, 0.889
and 0.967 respectively in diagnosing adenocarcinoma of cervix.
Synopsis
Adenocarcinoma comprises 25% of cervical cancers and has a bad prognosis and poor outcome of radiotherapy and chemical treatment in the advanced stage. Here, we report a radiomics method with multi-class texture features from semi-quantitative DCE-MRI maps to distinguish adenocarcinoma from squamous cell cancer. Multivariate models were trained on the training cohort and their performance was evaluated on the 5-fold cross-validation cohort using the area under ROC curve (AUC), accuracy, specificity and sensitivity. Our results showed the mean sensitivity, specificity, PPV, NPV and AUC were 0.96, 0.889, 0.967, 0.889 and 0.967 respectively in diagnosing adenocarcinoma of cervix.Introcuction
The two most common histologic subtypes of cervical cancer are squamous cell and adenocarcinoma, which account for about 70% and 25% respectively1. They have similar clinical signs and conventional MR imaging, whereas adenocarcinoma, which is less sensitive to radiotherapy and neoadjuvant chemical therapy, yields a higher recurrence and a poor prognosis2. Dynamic contrast-enhanced MRI (DCE-MRI) has been employed to evaluate the extent of tumor angiogenesis and tumor heterogeneity by analyzing patterns of enhancement. Many studies have explored heterogeneous enhancement patterns in DCE-MR images within the entire tumor to build predictive models of tumor subtypes based on the quantitative evaluation of contrast enhancement3. Texture analysis is a mathematical statistical procedure to extract objective and quantitative parameters (texture features) from given images. Several studies showed that texture features derived from DWI or DCE-MRI potentially predicted histological tumor differentiation and cancer stage4,5. Here, we report a potential method, DCE- MRI maps texture analysis combined with clinical index for evaluating cervical cancer. In this retrospective study, the first order and three higher order features (GLRM, GLRLM and GLSZM) were used for analyzing whole tumor DCE-MRI maps to investigate the value of distinguishing adenocarcinoma from squamous cell cancer in reference to histopathology results.Purpose
To determine the value of quantitative texture analysis of dynamic contrast-enhanced MRI maps of cervical carcinoma in the tumor subtypes prediction.Method
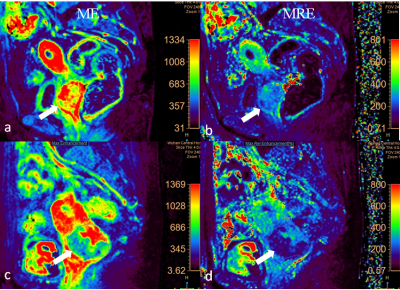
Thirty-nine patients with cervical carcinoma were enrolled in this retrospective, institutional review board (IRB)-approved study. DCE-MRI was performed on 3.0T scanner (Ingenia, Phillip Healthcare, Best, the Netherlands) by a 3D T1W High Resolution Isotropic Volume Examination (THRIVE) sequence with 36 phases. Co-occurrence matrix -based texture features were extracted from each tumor on maximum enhancement (ME) and maximum relative enhancement (MRE) maps from DCE-MRI using in-home radiomics tool based on Mat-Lab software. 3D volumes of interest (VOIs) of DCE-MRI maps comprising all ROIs encompassing the whole tumor were obtained. Multivariate models were trained on the training cohort and their performance was evaluated on the 5-fold cross-validation cohort using the area under ROC curve (AUC), accuracy, specificity and sensitivity. P value<0.05 was considered statistically significant.Results
Mean age of all 39 patients was 56.5±10.3 years. Histopathology revealed 9 adenocarcinoma and 30 squamous cell cancer; 7 well-differentiated, 21 moderately differentiated or moderately to poorly differentiated, and 11 poorly differentiated tumors; 7 FIGOⅠb, 18 FIGO Ⅱa, 8 FIGO Ⅱb, 6 FIGO Ⅲa, none of FIGO Ⅲb and Ⅳ. Lymph nodes were involved in 12 patients. On MRE maps, Skewness, variance, kurtosis, GLV (GLSZM), LAHE (GLSZM), HGZE (GLSZM), SAE (GLSZM), LGZE (GLSZM), SRGE (GLRLM), LRHGE (GLRLM), HGRE (GLRLM) and contrast (GLCM) of squamous cell cancer were statistically higher than that of adenocarcinoma (P<0.05),while LALE (GLSZM), SALE (GLSZM), LLRE (GLRLM), LRLGE (GLRLM) of squamous cell cancer were significantly lower than that of adenocarcinoma (P<0.05). On ME maps, only kurtosis of squamous cell cancer was statistically higher than that of adenocarcinoma (P<0.05). Our results using a multivariable logistic regression analysis showed the mean sensitivity, specificity, PPV, NPV and AUC were 0.96, 0.889, 0.967, 0.889 and 0.967 respectively in diagnosing adenocarcinoma of cervix.Conclusion and discussion
Volumetric texture analysis of ME and MRE maps holds a potential tool in distinguish adenocarcinoma from squamous cell cervical cancer. In this study, we used the first order and three higher order features (GLRM, GLRLM and GLSZM) including 1765 features for analyzing whole tumor DCE-MRI maps. Interestingly, more texture features from MRE maps showed significant differences in comparison to those from ME maps, it may be mainly explained by two aspects: heterogeneous and varied enhanced patterns in intratumoral regions correlated with clinical and histologic features. Squamous cell cervical cancer often enhances as type Ⅰ or type Ⅱ, whereas adenocarcinoma enhances as type Ⅲ. The cross-model validation in the present study showed a relatively high accuracy, indicating that the large number of support vectors may simply reflect the considerable variation in tumor characteristics among the patients, however, the statistical power was limited due to the relatively small number of samples. Further research will be necessary to verify our preliminary findings in a larger cohort.Acknowledgements
No acknowledgement found.References
1.Lea JS, Lin KY. Cervical cancer. Obstetrics and gynecology clinics of North America. 2012;39(2):233-253. 2.Noh JM, Park W, Kim YS, Kim JY, Kim HJ, Kim J, et al. Comparison of clinical outcomes of adenocarcinoma and adenosquamous carcinoma in uterine cervical cancer patients receiving surgical resection followed by radiotherapy: a multicenter retrospective study (KROG 13-10). Gynecologic oncology. 2014;132(3):618-623.
3.M. A. Zahra, K. G. Hollingsworth, E. Sala, D. J.Lomas, and L. T. Tan, “Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy,” Lancet Oncology,2007;8(1): 63-74.
4.Guan Y, Li W, Jiang Z, Zhang B, Chen Y, Huang X, et al. Value of whole-lesion apparent diffusion coefficient (ADC) first-order statistics and texture features in clinical staging of cervical cancers. Clinical radiology. 2017;72(11):951-958.
5.Torheim T, Malinen E, Kvaal K, Lyng H, Indahl UG, Andersen EK, et al. Classification of dynamic contrast enhanced MR images of cervical cancers using texture analysis and support vector machines. IEEE transactions on medical imaging. 2014;33(8):1648-1656.
Figures